Crystal structure of the bromodomain of human Bromodomain and PHD finger-containing Transcription Factor (BPTF) bound to a potent inhibitor
Balikci, E., Ni, X., Bountra, C., von Delft, F., Huber, K.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Nucleosome-remodeling factor subunit BPTF | 123 | Homo sapiens | Mutation(s): 0 Gene Names: BPTF, FAC1, FALZ | 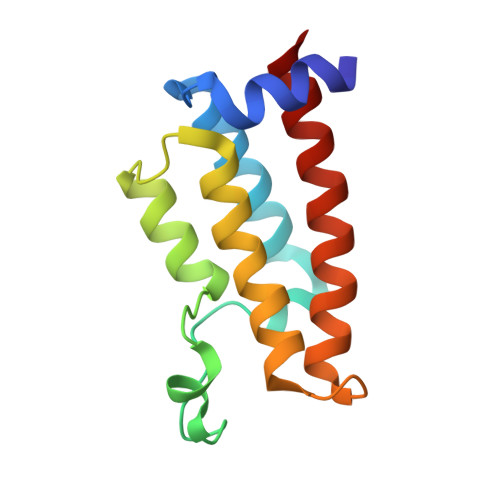 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q12830 (Homo sapiens) Explore Q12830 Go to UniProtKB: Q12830 | |||||
PHAROS: Q12830 GTEx: ENSG00000171634 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q12830 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 4 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| W2W (Subject of Investigation/LOI) Query on W2W | C [auth A], H [auth B] | 7-(1-cyclopropylpyrazol-4-yl)-2-[[2-fluoranyl-4-(4-methylpiperazin-1-yl)sulfonyl-phenyl]amino]-3-methyl-pyrido[1,2-a]pyrimidin-4-one C26 H28 F N7 O3 S JYTISQGEFSHUIR-UHFFFAOYSA-N |  | ||
| GOL Query on GOL | D [auth A], E [auth A] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| DMS Query on DMS | L [auth B] | DIMETHYL SULFOXIDE C2 H6 O S IAZDPXIOMUYVGZ-UHFFFAOYSA-N |  | ||
| EDO Query on EDO | F [auth A], G [auth B], I [auth B], J [auth B], K [auth B] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 58.277 | α = 90 |
| b = 27.043 | β = 93.48 |
| c = 77.637 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| XDS | data reduction |
| STARANISO | data scaling |
| MOLREP | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Innovative Medicines Initiative | Switzerland | 875510 |