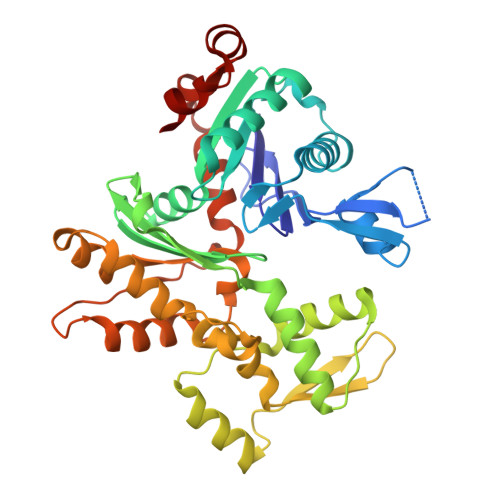
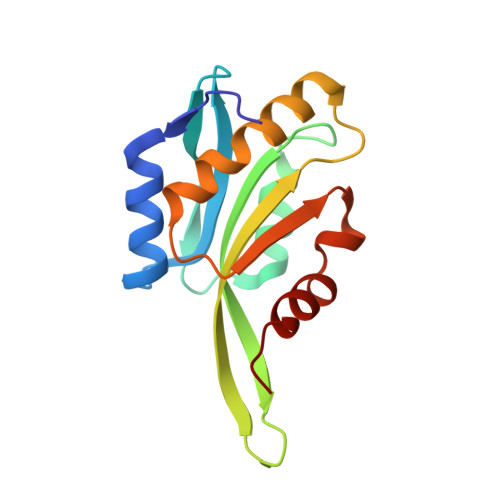
CryoET shows cofilactin filaments inside the microtubule lumen.
Santos, C.V., Rogers, S.L., Carter, A.P.(2023) bioRxiv
- PubMed: 37034688
- DOI: https://doi.org/10.1101/2023.03.31.535077
- Primary Citation of Related Structures:
8OH4 - PubMed Abstract:
Cytoplasmic microtubules are tubular polymers that can harbor small proteins or filaments inside their lumen. The identity of these objects and what causes their accumulation has not been conclusively established. Here, we used cryogenic electron tomography (cryoET) of Drosophila S2 cell protrusions and found filaments inside the microtubule lumen, which resemble those reported recently in human HAP1 cells. The frequency of these filaments increased upon inhibition of the sarco/endoplasmic reticulum Ca 2+ ATPase (SERCA) with the small-molecule drug thapsigargin. Subtomogram averaging showed that the luminal filaments adopt a helical structure reminiscent of cofilin-bound actin (cofilactin). Consistent with this, cofilin was activated in cells under the same conditions that increased luminal filament occurrence. Furthermore, RNAi knock-down of cofilin reduced the frequency of luminal filaments with cofilactin morphology. These results suggest that cofilin activation stimulates its accumulation on actin filaments inside the microtubule lumen.
- MRC Laboratory of Molecular Biology, Francis Crick Ave, Cambridge, CB2 0QH, UK.
Organizational Affiliation: