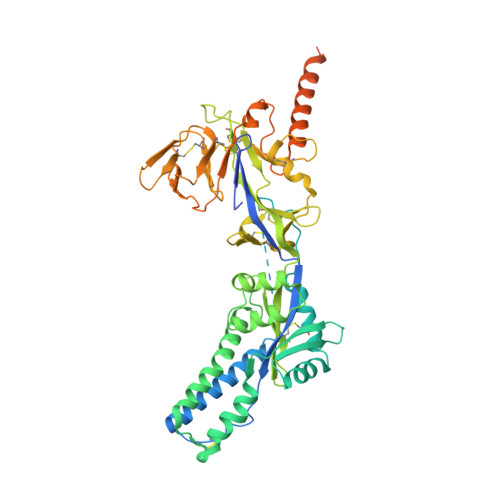
Farnesyltransferase inhibitor lonafarnib suppresses respiratory syncytial virus infection by blocking conformational change of fusion glycoprotein.
Yang, Q., Xue, B., Liu, F., Lu, Y., Tang, J., Yan, M., Wu, Q., Chen, R., Zhou, A., Liu, L., Liu, J., Qu, C., Wu, Q., Fu, M., Zhong, J., Dong, J., Chen, S., Wang, F., Zhou, Y., Zheng, J., Peng, W., Shang, J., Chen, X.(2024) Signal Transduct Target Ther 9: 144-144
- PubMed: 38853183
- DOI: https://doi.org/10.1038/s41392-024-01858-5
- Primary Citation of Related Structures:
8KG5 - PubMed Abstract:
Respiratory syncytial virus (RSV) is the major cause of bronchiolitis and pneumonia in young children and the elderly. There are currently no approved RSV-specific therapeutic small molecules available. Using high-throughput antiviral screening, we identified an oral drug, the prenylation inhibitor lonafarnib, which showed potent inhibition of the RSV fusion process. Lonafarnib exhibited antiviral activity against both the RSV A and B genotypes and showed low cytotoxicity in HEp-2 and human primary bronchial epithelial cells (HBEC). Time-of-addition and pseudovirus assays demonstrated that lonafarnib inhibits RSV entry, but has farnesyltransferase-independent antiviral efficacy. Cryo-electron microscopy revealed that lonafarnib binds to a triple-symmetric pocket within the central cavity of the RSV F metastable pre-fusion conformation. Mutants at the RSV F sites interacting with lonafarnib showed resistance to lonafarnib but remained fully sensitive to the neutralizing monoclonal antibody palivizumab. Furthermore, lonafarnib dose-dependently reduced the replication of RSV in BALB/c mice. Collectively, lonafarnib could be a potential fusion inhibitor for RSV infection.
- Guangzhou National Laboratory, Guangzhou, 510005, China.
Organizational Affiliation: