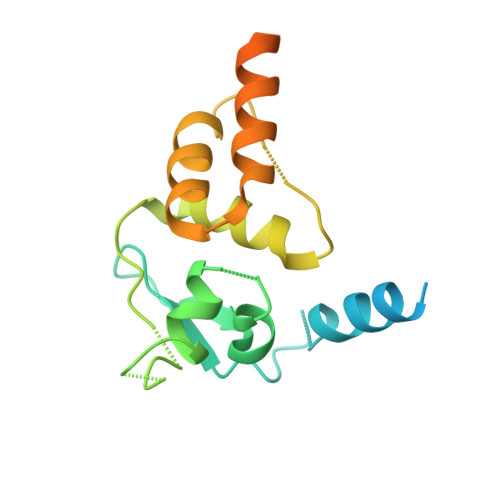
A conserved N-terminal motif of CUL3 contributes to assembly and E3 ligase activity of CRL3 KLHL22.
Wang, W., Liang, L., Dai, Z., Zuo, P., Yu, S., Lu, Y., Ding, D., Chen, H., Shan, H., Jin, Y., Mao, Y., Yin, Y.(2024) Nat Commun 15: 3789-3789
- PubMed: 38710693
- DOI: https://doi.org/10.1038/s41467-024-48045-2
- Primary Citation of Related Structures:
8K8T, 8K9I - PubMed Abstract:
The CUL3-RING E3 ubiquitin ligases (CRL3s) play an essential role in response to extracellular nutrition and stress stimuli. The ubiquitin ligase function of CRL3s is activated through dimerization. However, how and why such a dimeric assembly is required for its ligase activity remains elusive. Here, we report the cryo-EM structure of the dimeric CRL3 KLHL22 complex and reveal a conserved N-terminal motif in CUL3 that contributes to the dimerization assembly and the E3 ligase activity of CRL3 KLHL22 . We show that deletion of the CUL3 N-terminal motif impairs dimeric assembly and the E3 ligase activity of both CRL3 KLHL22 and several other CRL3s. In addition, we found that the dynamics of dimeric assembly of CRL3 KLHL22 generates a variable ubiquitination zone, potentially facilitating substrate recognition and ubiquitination. These findings demonstrate that a CUL3 N-terminal motif participates in the assembly process and provide insights into the assembly and activation of CRL3s.
- Institute of Precision Medicine, Peking University Shenzhen Hospital, Shenzhen, 518036, China.
Organizational Affiliation: