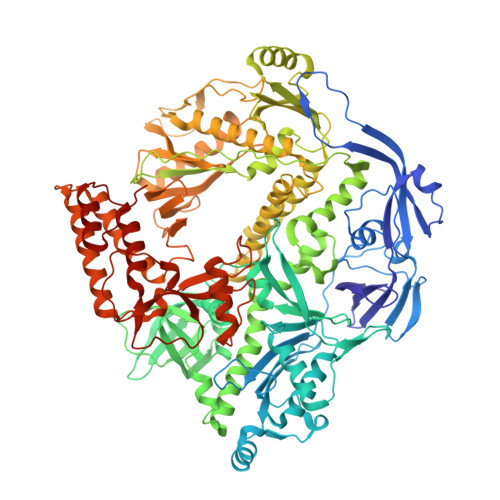
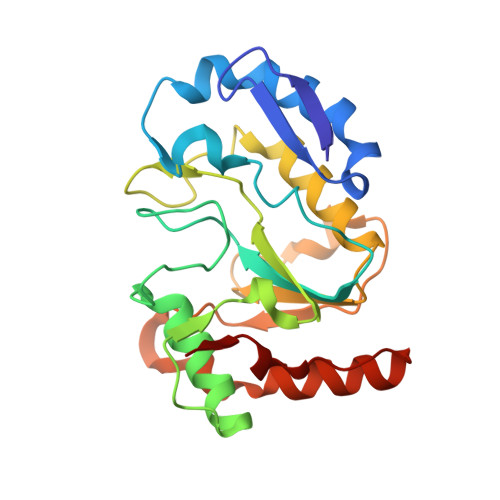
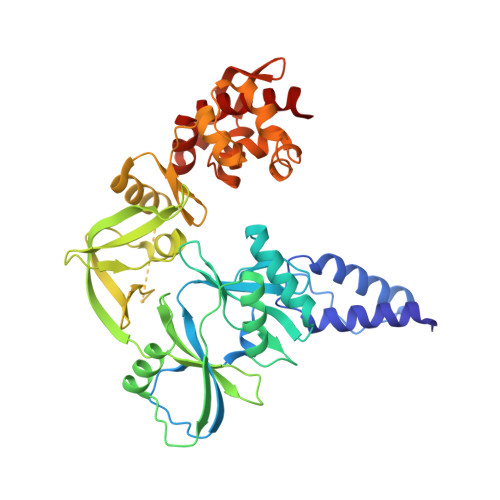
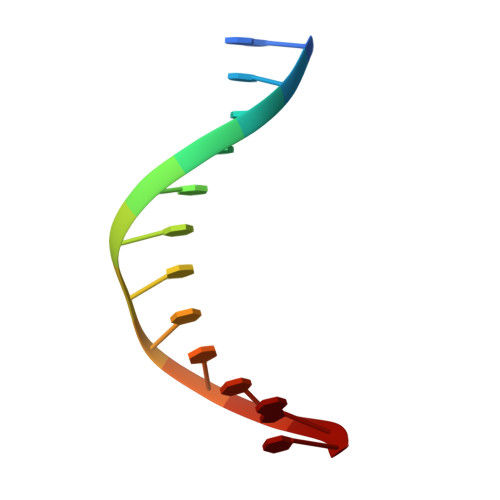
Structural basis for the inhibition mechanism of the DNA polymerase holoenzyme from mpox virus.
Shen, Y., Li, Y., Yan, R.(2024) Structure 32: 654-661.e3
- PubMed: 38579705
- DOI: https://doi.org/10.1016/j.str.2024.03.004
- Primary Citation of Related Structures:
8K8S, 8K8U - PubMed Abstract:
There are three key components at the core of the mpox virus (MPXV) DNA polymerase holoenzyme: DNA polymerase F8, processivity factors A22, and the Uracil-DNA glycosylase E4. The holoenzyme is recognized as a vital antiviral target because MPXV replicates in the cytoplasm of host cells. Nucleotide analogs such as cidofovir and cytarabine (Ara-C) have shown potential in curbing MPXV replication and they also display promise against other poxviruses. However, the mechanism behind their inhibitory effects remains unclear. Here, we present the cryo-EM structure of the DNA polymerase holoenzyme F8/A22/E4 bound with its competitive inhibitor Ara-C-derived cytarabine triphosphate (Ara-CTP) at an overall resolution of 3.0 Å and reveal its inhibition mechanism. Ara-CTP functions as a direct chain terminator in proximity to the deoxycytidine triphosphate (dCTP)-binding site. The extra hydrogen bond formed with Asn665 makes it more potent in binding than dCTP. Asn665 is conserved among eukaryotic B-family polymerases.
- Center for Infectious Disease Research, Westlake Laboratory of Life Sciences and Biomedicine, Key Laboratory of Structural Biology of Zhejiang Province, School of Life Sciences, Westlake University, Hangzhou 310024, Zhejiang Province, China.
Organizational Affiliation: