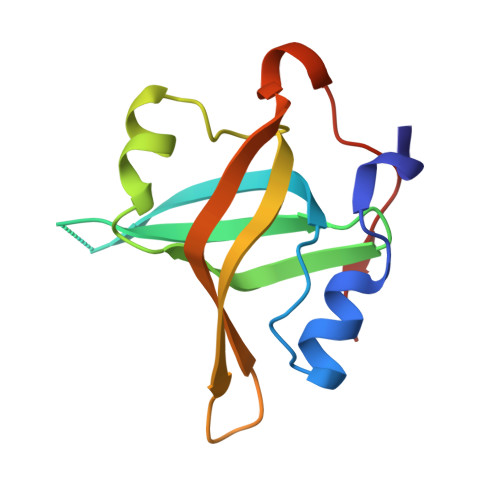
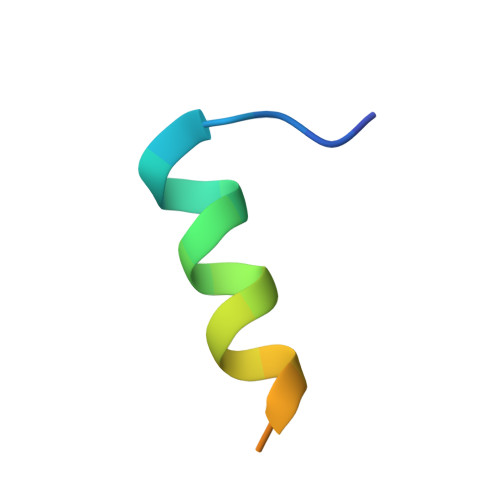
Structural characterization of human RPA70N association with DNA damage response proteins.
Wu, Y., Fu, W., Zang, N., Zhou, C.(2023) Elife 12
- PubMed: 37668474
- DOI: https://doi.org/10.7554/eLife.81639
- Primary Citation of Related Structures:
7XUT, 7XUV, 7XUW, 7XV0, 7XV1, 7XV4, 8JZV, 8JZY, 8K00 - PubMed Abstract:
The heterotrimeric Replication protein A (RPA) is the ubiquitous eukaryotic single-stranded DNA (ssDNA) binding protein and participates in nearly all aspects of DNA metabolism, especially DNA damage response. The N-terminal OB domain of the RPA70 subunit (RPA70N) is a major protein-protein interaction element for RPA and binds to more than 20 partner proteins. Previous crystallography studies of RPA70N with p53, DNA2 and PrimPol fragments revealed that RPA70N binds to amphipathic peptides that mimic ssDNA. NMR chemical-shift studies also provided valuable information on the interaction of RPA70N residues with target sequences. However, it is still unclear how RPA70N recognizes and distinguishes such a diverse group of target proteins. Here, we present high-resolution crystal structures of RPA70N in complex with peptides from eight DNA damage response proteins. The structures show that, in addition to the ssDNA mimicry mode of interaction, RPA70N employs multiple ways to bind its partners. Our results advance the mechanistic understanding of RPA70N-mediated recruitment of DNA damage response proteins.
- School of Public Health & Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Zhejiang, China.
Organizational Affiliation: