Cryo-EM Structure of MapSPARTA
Huang, P.P., Guo, L.J., Li, Z.X., Yan, P.R., Chen, M.R., Xiao, Y.B.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Piwi domain-containing protein | E [auth A], F [auth C] | 507 | Maribacter polysiphoniae | Mutation(s): 0 Gene Names: LX92_01809 | 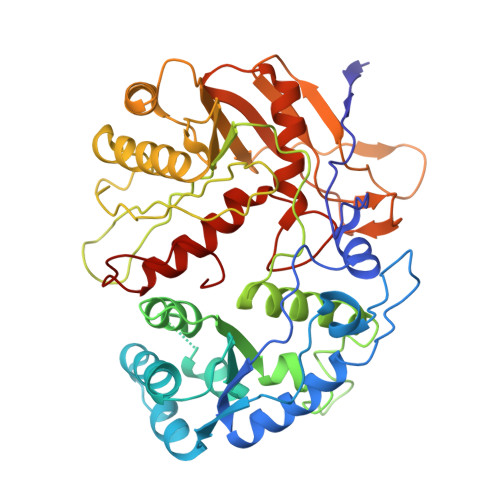 |
UniProt | |||||
Find proteins for A0A316E3U6 (Maribacter polysiphoniae) Explore A0A316E3U6 Go to UniProtKB: A0A316E3U6 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0A316E3U6 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 4 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| TIR domain-containing protein | G [auth D], H [auth B] | 452 | Maribacter polysiphoniae | Mutation(s): 0 Gene Names: LX92_01810 | 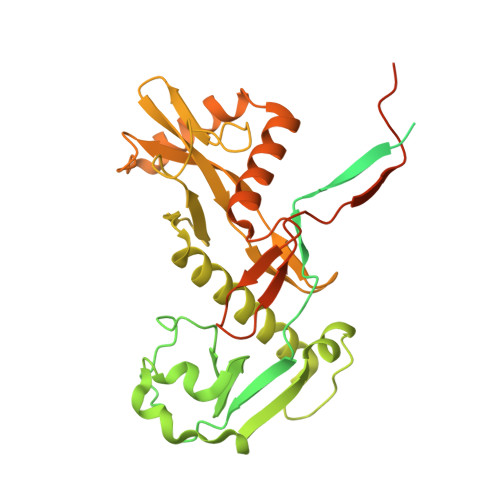 |
UniProt | |||||
Find proteins for A0A316E683 (Maribacter polysiphoniae) Explore A0A316E683 Go to UniProtKB: A0A316E683 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0A316E683 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar nucleic acids by: Sequence | 3D Structure
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| RNA (5'-R(P*UP*GP*AP*CP*GP*GP*CP*UP*CP*UP*AP*AP*UP*CP*UP*AP*UP*UP*AP*GP*U)-3') | A [auth E], C [auth G] | 21 | Maribacter polysiphoniae | 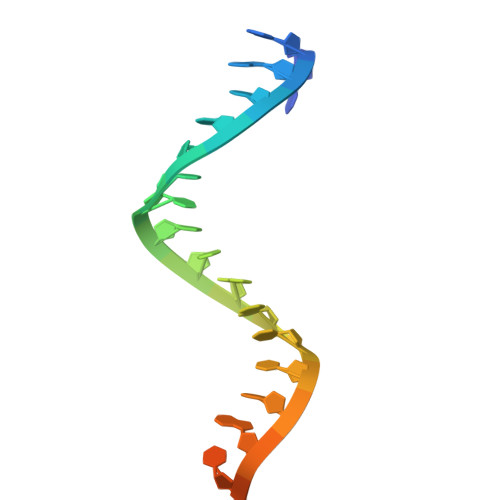 | |
Sequence AnnotationsExpand | |||||
| |||||
Find similar nucleic acids by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| DNA (25-MER) | B [auth F], D [auth H] | 25 | Maribacter polysiphoniae | 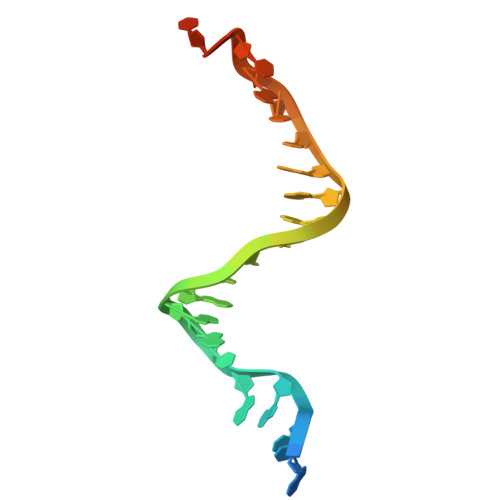 | |
Sequence AnnotationsExpand | |||||
| |||||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Ministry of Science and Technology (MoST, China) | China | Nos.2018YFA0902000 |
| Ministry of Science and Technology (MoST, China) | China | ZX-2021ZD0203404 |
| National Natural Science Foundation of China (NSFC) | China | Nos. 32271330 |
| National Natural Science Foundation of China (NSFC) | China | Nos. 32000889 |
| National Natural Science Foundation of China (NSFC) | China | Nos. 31970547 |