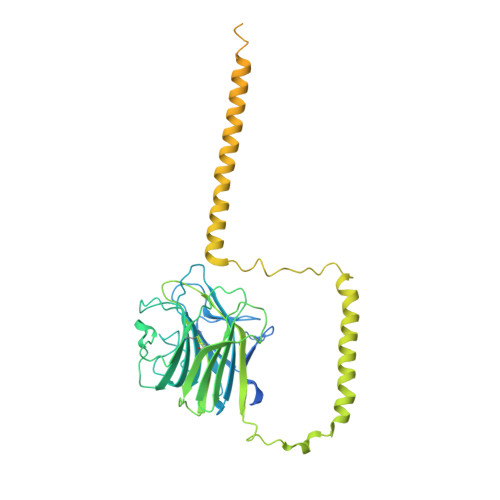
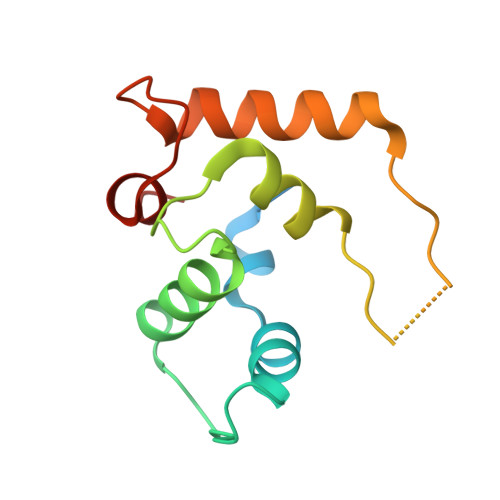
Structure of full-length ERGIC-53 in complex with MCFD2 for cargo transport.
Watanabe, S., Kise, Y., Yonezawa, K., Inoue, M., Shimizu, N., Nureki, O., Inaba, K.(2024) Nat Commun 15: 2404-2404
- PubMed: 38493152
- DOI: https://doi.org/10.1038/s41467-024-46747-1
- Primary Citation of Related Structures:
8JP4, 8JP5, 8JP6, 8JP7, 8JP8, 8JP9, 8JPG - PubMed Abstract:
ERGIC-53 transports certain subsets of newly synthesized secretory proteins and membrane proteins from the endoplasmic reticulum to the Golgi apparatus. Despite numerous structural and functional studies since its identification, the overall architecture and mechanism of action of ERGIC-53 remain unclear. Here we present cryo-EM structures of full-length ERGIC-53 in complex with its functional partner MCFD2. These structures reveal that ERGIC-53 exists as a homotetramer, not a homohexamer as previously suggested, and comprises a four-leaf clover-like head and a long stalk composed of three sets of four-helix coiled-coil followed by a transmembrane domain. 3D variability analysis visualizes the flexible motion of the long stalk and local plasticity of the head region. Notably, MCFD2 is shown to possess a Zn 2+ -binding site in its N-terminal lid, which appears to modulate cargo binding. Altogether, distinct mechanisms of cargo capture and release by ERGIC- 53 via the stalk bending and metal binding are proposed.
- Institute of Multidisciplinary Research for Advanced Materials, Tohoku University, Sendai, Miyagi, 980-8577, Japan. satoshi.watanabe.c1@tohoku.ac.jp.
Organizational Affiliation: