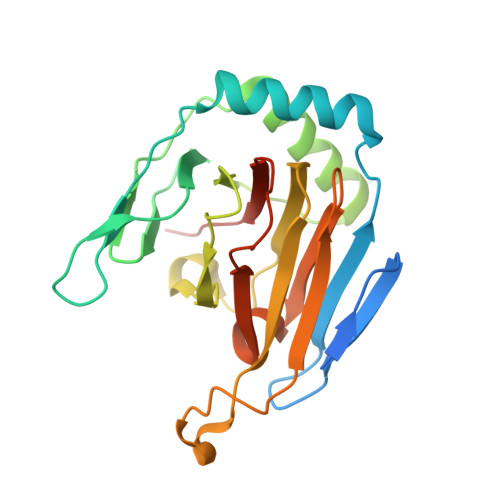
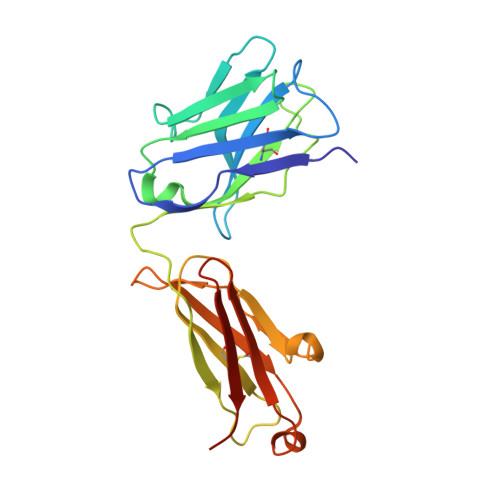
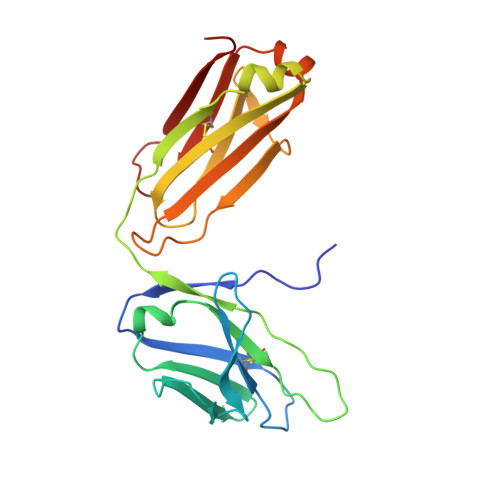
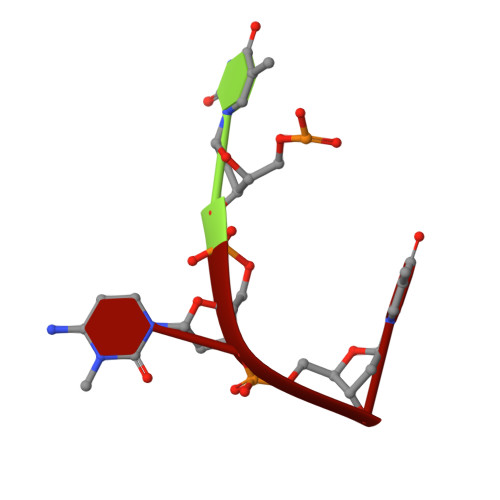
The Molecular Basis of Human ALKBH3 Mediated RNA N 1 -methyladenosine (m 1 A) Demethylation.
Zhang, L., Duan, H.C., Paduch, M., Hu, J., Zhang, C., Mu, Y., Lin, H., He, C., Kossiakoff, A.A., Jia, G., Zhang, L.(2024) Angew Chem Int Ed Engl 63
- PubMed: 38158383
- DOI: https://doi.org/10.1002/anie.202313900
- Primary Citation of Related Structures:
8JNK, 8JNR - PubMed Abstract:
N 1 -methyladenosine (m 1 A) is a prevalent post-transcriptional RNA modification, and the distribution and dynamics of the modification play key epitranscriptomic roles in cell development. At present, the human AlkB Fe(II)/α-ketoglutarate-dependent dioxygenase family member ALKBH3 is the only known mRNA m 1 A demethylase, but its catalytic mechanism remains unclear. Here, we present the structures of ALKBH3-oligo crosslinked complexes obtained with the assistance of a synthetic antibody crystallization chaperone. Structural and biochemical results showed that ALKBH3 utilized two β-hairpins (β4-loop-β5 and β'-loop-β'') and the α2 helix to facilitate single-stranded substrate binding. Moreover, a bubble-like region around Asp194 and a key residue inside the active pocket (Thr133) enabled specific recognition and demethylation of m 1 A- and 3-methylcytidine (m 3 C)-modified substrates. Mutation of Thr133 to the corresponding residue in the AlkB Fe(II)/α-ketoglutarate-dependent dioxygenase family members FTO or ALKBH5 converted ALKBH3 substrate selectivity from m 1 A to N 6 -methyladenosine (m 6 A), as did Asp194 deletion. Our findings provide a molecular basis for understanding the mechanisms of substrate recognition and m 1 A demethylation by ALKBH3. This study is expected to aid structure-guided design of chemical probes for further functional studies and therapeutic applications.
- Department of Pharmacology and Chemical Biology, State Key Laboratory of Systems Medicine for Cancer, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China.
Organizational Affiliation: