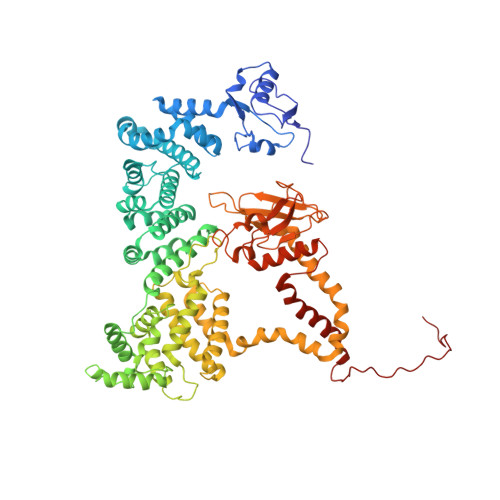
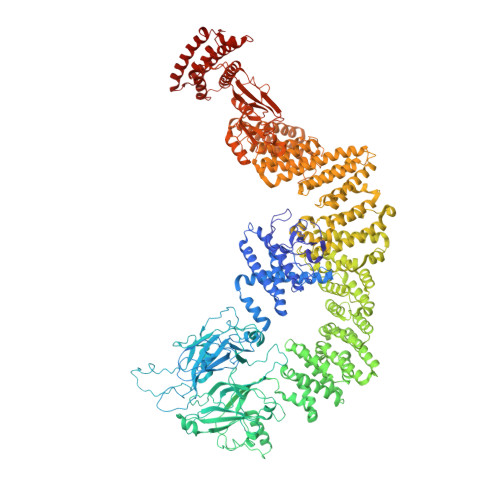
RhoG facilitates a conformational transition in the guanine nucleotide exchange factor complex DOCK5/ELMO1 to an open state.
Kukimoto-Niino, M., Katsura, K., Ishizuka-Katsura, Y., Mishima-Tsumagari, C., Yonemochi, M., Inoue, M., Nakagawa, R., Kaushik, R., Zhang, K.Y.J., Shirouzu, M.(2024) J Biological Chem 300: 107459-107459
- PubMed: 38857861
- DOI: https://doi.org/10.1016/j.jbc.2024.107459
- Primary Citation of Related Structures:
8JHK, 8XM7, 8ZJ2, 8ZJI, 8ZJJ, 8ZJK, 8ZJL, 8ZJM - PubMed Abstract:
The dedicator of cytokinesis (DOCK)/engulfment and cell motility (ELMO) complex serves as a guanine nucleotide exchange factor (GEF) for the GTPase Rac. RhoG, another GTPase, activates the ELMO-DOCK-Rac pathway during engulfment and migration. Recent cryo-EM structures of the DOCK2/ELMO1 and DOCK2/ELMO1/Rac1 complexes have identified closed and open conformations that are key to understanding the autoinhibition mechanism. Nevertheless, the structural details of RhoG-mediated activation of the DOCK/ELMO complex remain elusive. Herein, we present cryo-EM structures of DOCK5/ELMO1 alone and in complex with RhoG and Rac1. The DOCK5/ELMO1 structure exhibits a closed conformation similar to that of DOCK2/ELMO1, suggesting a shared regulatory mechanism of the autoinhibitory state across DOCK-A/B subfamilies (DOCK1-5). Conversely, the RhoG/DOCK5/ELMO1/Rac1 complex adopts an open conformation that differs from that of the DOCK2/ELMO1/Rac1 complex, with RhoG binding to both ELMO1 and DOCK5. The alignment of the DOCK5 PIP3 binding site with the RhoG C-terminal lipidation site suggests simultaneous binding of RhoG and DOCK5/ELMO1 to the plasma membrane. Structural comparison of the apo and RhoG-bound states revealed that RhoG facilitates a closed-to-open state conformational change of DOCK5/ELMO1. Biochemical and surface plasmon resonance (SPR) assays confirm that RhoG enhances the Rac GEF activity of DOCK5/ELMO1 and increases its binding affinity for Rac1. Further analysis of structural variability underscored the conformational flexibility of the DOCK5/ELMO1/Rac1 complex core, potentially facilitating the proximity of the DOCK5 GEF domain to the plasma membrane. These findings elucidate the structural mechanism underlying the RhoG-induced allosteric activation and membrane binding of the DOCK/ELMO complex.
- RIKEN Center for Biosystems Dynamics Research, 1-7-22 Suehiro-cho, Tsurumi-ku, Yokohama, Kanagawa 230-0045, Japan. Electronic address: kukimoto@riken.jp.
Organizational Affiliation: