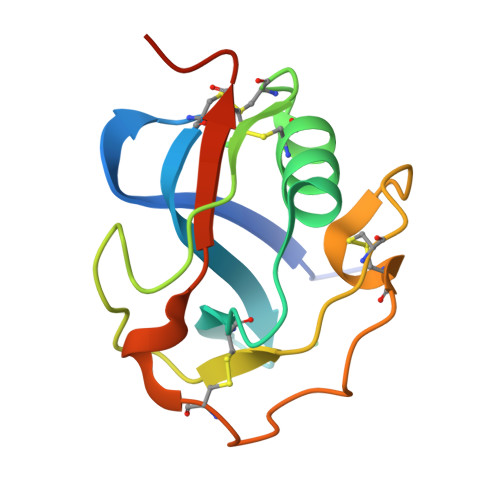
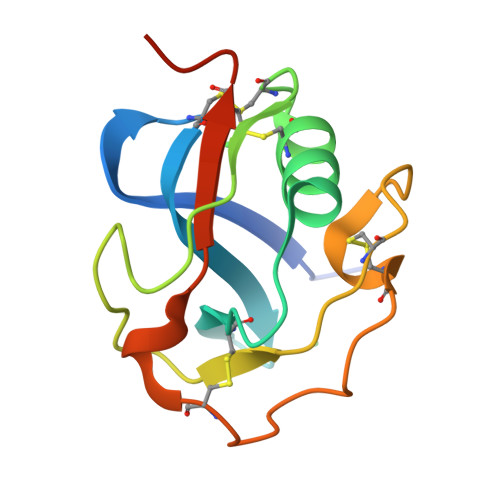
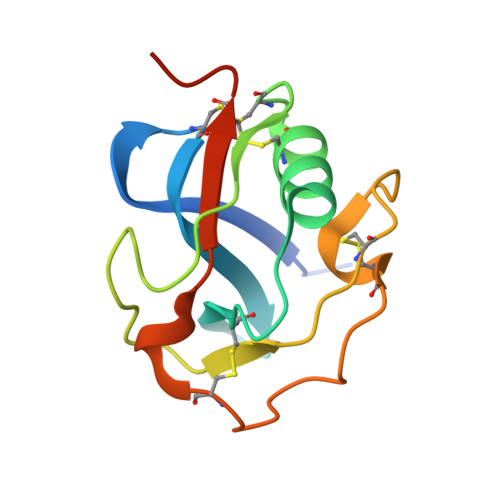
Refolding, Crystallization, and Crystal Structure Analysis of a Scavenger Receptor Cysteine-Rich Domain of Human Salivary Agglutinin Expressed in Escherichia coli.
Zhang, C., Lu, P., Wei, S., Hu, C., Miyoshi, M., Okamoto, K., Itoh, H., Okuda, S., Suzuki, M., Kawakami, H., Nagata, K.(2024) Protein J 43: 283-297
- PubMed: 38265733
- DOI: https://doi.org/10.1007/s10930-023-10173-x
- Primary Citation of Related Structures:
8J8D, 8WZS - PubMed Abstract:
Scavenger receptors are a protein superfamily that typically consists of one or more repeats of the scavenger receptor cysteine-rich structural domain (SRCRD), which is an ancient and highly conserved protein module. The expression and purification of eukaryotic proteins containing multiple disulfide bonds has always been challenging. The expression systems that are commonly used to express SRCRD proteins mainly consist of eukaryotic protein expression systems. Herein, we established a high-level expression strategy of a Type B SRCRD unit from human salivary agglutinin using the Escherichia coli expression system, followed by a refolding and purification process. The untagged recombinant SRCRD was expressed in E. coli using the pET-32a vector, which was followed by a refolding process using the GSH/GSSG redox system. The SRCRD expressed in E. coli SHuffle T7 showed better solubility after refolding than that expressed in E. coli BL21(DE3), suggesting the importance of the disulfide bond content prior to refolding. The quality of the refolded protein was finally assessed using crystallization and crystal structure analysis. As proteins refolded from inclusion bodies exhibit a high crystal quality and reproducibility, this method is considered a reliable strategy for SRCRD protein expression and purification. To further confirm the structural integrity of the refolded SRCRD protein, the purified protein was subjected to crystallization using sitting-drop vapor diffusion method. The obtained crystals of SRCRD diffracted X-rays to a resolution of 1.47 Å. The solved crystal structure appeared to be highly conserved, with four disulfide bonds appropriately formed. The surface charge distribution of homologous SRCRD proteins indicates that the negatively charged region at the surface is associated with their calcium-dependent ligand recognition. These results suggest that a high-quality SRCRD protein expressed by E. coli SHuffle T7 can be successfully folded and purified, providing new options for the expression of members of the scavenger receptor superfamily.
Organizational Affiliation:
Department of Applied Biological Chemistry, Graduate School of Agricultural and Life Sciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo, 113-8657, Japan.