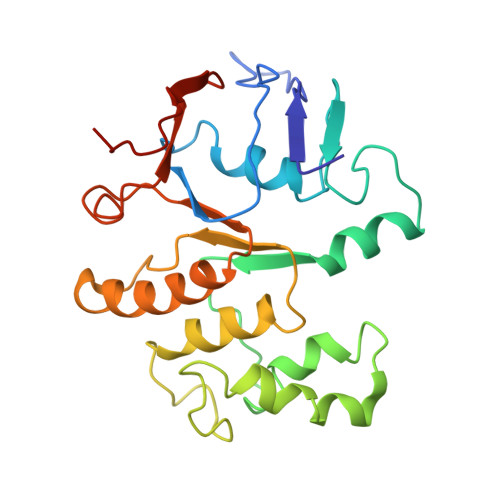
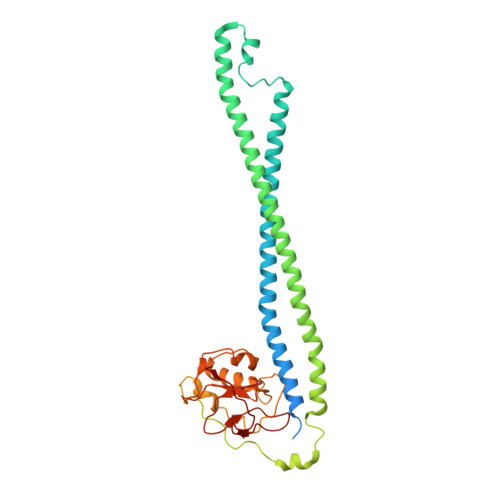
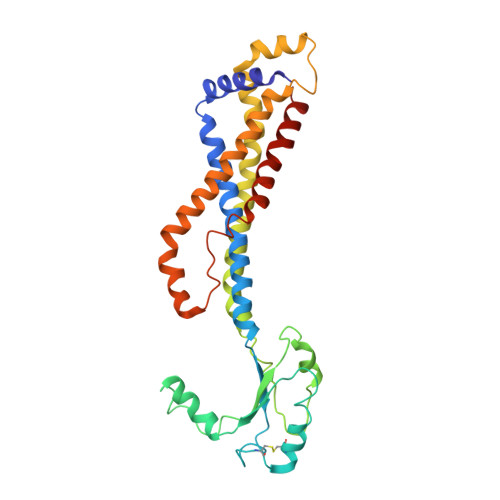
Regulation of the cell division hydrolase RipC by the FtsEX system in Mycobacterium tuberculosis.
Li, J., Xu, X., Shi, J., Hermoso, J.A., Sham, L.T., Luo, M.(2023) Nat Commun 14: 7999-7999
- PubMed: 38044344
- DOI: https://doi.org/10.1038/s41467-023-43770-6
- Primary Citation of Related Structures:
8IDB, 8IDC, 8IDD, 8IGQ, 8JIA - PubMed Abstract:
The FtsEX complex regulates, directly or via a protein mediator depending on bacterial genera, peptidoglycan degradation for cell division. In mycobacteria and Gram-positive bacteria, the FtsEX system directly activates peptidoglycan-hydrolases by a mechanism that remains unclear. Here we report our investigation of Mycobacterium tuberculosis FtsEX as a non-canonical regulator with high basal ATPase activity. The cryo-EM structures of the FtsEX system alone and in complex with RipC, as well as the ATP-activated state, unveil detailed information on the signal transduction mechanism, leading to the activation of RipC. Our findings indicate that RipC is recognized through a "Match and Fit" mechanism, resulting in an asymmetric rearrangement of the extracellular domains of FtsX and a unique inclined binding mode of RipC. This study provides insights into the molecular mechanisms of FtsEX and RipC regulation in the context of a critical human pathogen, guiding the design of drugs targeting peptidoglycan remodeling.
- Department of Biological Sciences, Faculty of Science, National University of Singapore, Singapore, Singapore.
Organizational Affiliation: