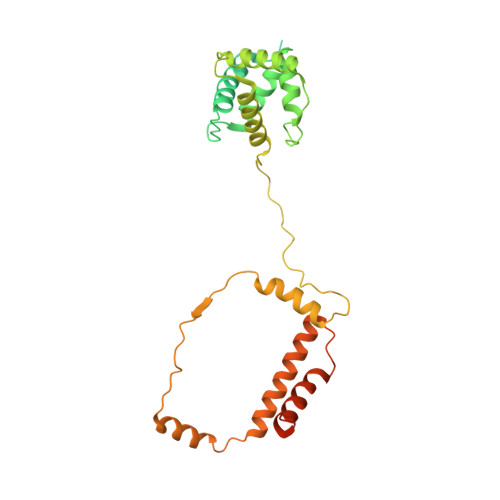
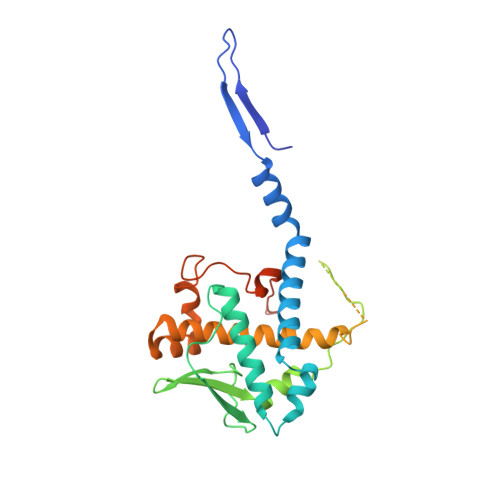
Architecture of the baculovirus nucleocapsid revealed by cryo-EM.
Jia, X., Gao, Y., Huang, Y., Sun, L., Li, S., Li, H., Zhang, X., Li, Y., He, J., Wu, W., Venkannagari, H., Yang, K., Baker, M.L., Zhang, Q.(2023) Nat Commun 14: 7481-7481
- PubMed: 37980340
- DOI: https://doi.org/10.1038/s41467-023-43284-1
- Primary Citation of Related Structures:
8I8A, 8I8B, 8I8C - PubMed Abstract:
Baculovirus Autographa californica multiple nucleopolyhedrovirus (AcMNPV) has been widely used as a bioinsecticide and a protein expression vector. Despite their importance, very little is known about the structure of most baculovirus proteins. Here, we show a 3.2 Å resolution structure of helical cylindrical body of the AcMNPV nucleocapsid, composed of VP39, as well as 4.3 Å resolution structures of both the head and the base of the nucleocapsid composed of over 100 protein subunits. AcMNPV VP39 demonstrates some features of the HK97-like fold and utilizes disulfide-bonds and a set of interactions at its C-termini to mediate nucleocapsid assembly and stability. At both ends of the nucleocapsid, the VP39 cylinder is constricted by an outer shell ring composed of proteins AC104, AC142 and AC109. AC101(BV/ODV-C42) and AC144(ODV-EC27) form a C14 symmetric inner layer at both capsid head and base. In the base, these proteins interact with a 7-fold symmetric capsid plug, while a portal-like structure is seen in the central portion of head. Additionally, we propose an application of AlphaFold2 for model building in intermediate resolution density.
- State key laboratory of biocontrol, School of Life Sciences, Sun Yat-sen University, 510275, Guangzhou, China.
Organizational Affiliation: