Crystal Structures of DNA-bound Fish IRF10 and IRF11 Reveal the Determinants of IFN Regulation.
Wang, Z.X., Liu, B., Xie, H., Liu, X., Li, X., Shi, F., Ouyang, S., Zhang, Y.A.(2024) J Immunol 213: 743-752
- PubMed: 39058321
- DOI: https://doi.org/10.4049/jimmunol.2300414
- Primary Citation of Related Structures:
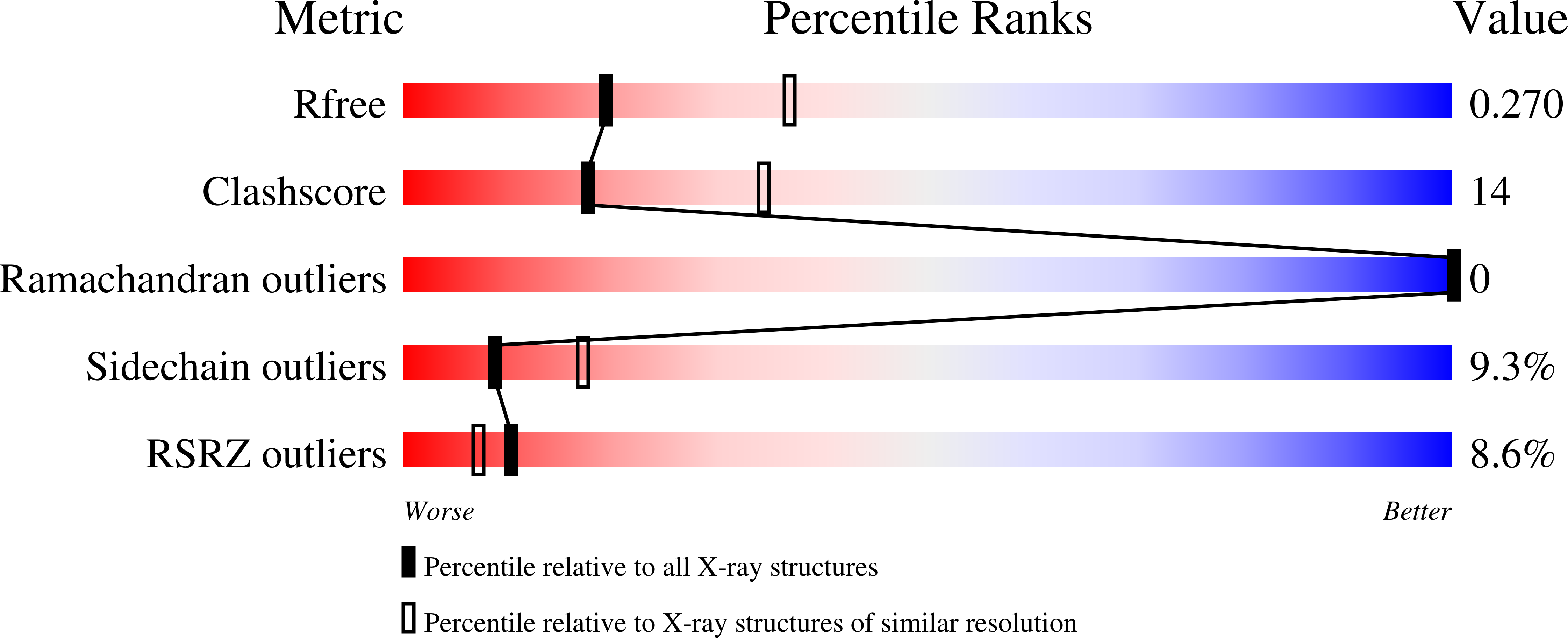
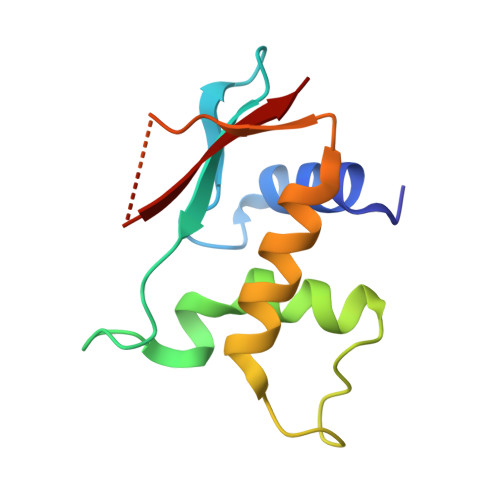
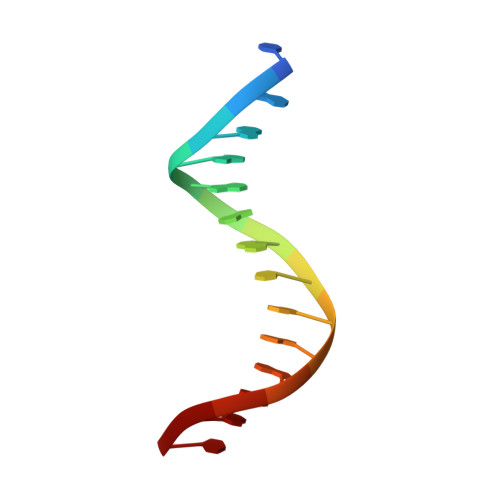
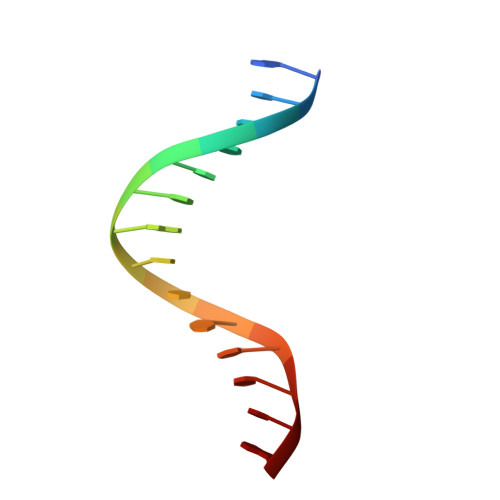
8HCL, 8HCM, 8HCS - PubMed Abstract:
IFN regulatory factors (IRFs) are transcription factors that mediate homeostatic mechanisms of host defense against pathogens. In addition to IRF1-9, which are conserved across vertebrates, teleost fishes have two other IRFs, IRF10 and IRF11. In zebrafish (Danio rerio), IRF10 represses the expression of IFNφ1 and IFNφ3, whereas IRF11 exerts the opposite effect. In this study, we found IRF10 could significantly inhibit the expression of IFNφ1 and IFNφ3 induced by IFN11 to synergistically regulate type I IFN expression. To clarify the synergistically regulatory mechanism of IRF10 and IRF11 in type I IFN expression, we determined and analyzed the crystal structures of the DNA-binding domains (DBDs) of zebrafish IRF10 and IRF11 bound to DNA, as well as IRF11 DBD in apo form. The interactions of IRF10-DBD and IRF11-DBD with DNA backbone were elaborated in detail. Further analysis showed that IRF10 and IRF11 have the same binding patterns and comparable affinities with the IFN-sensitive response elements of IFNφ1 and IFNφ3 promoters. Therefore, IRF10 could function as a controlling factor for IRF11 by competitive binding of the IFN-sensitive response elements to coregulate the host IFN response. Accordingly, similar to IRF1 and IRF2 in mammals, IRF10 and IRF11 act as another pair of negative and positive regulators to balance the antiviral responses in fish.
- State Key Laboratory of Agricultural Microbiology, Hubei Hongshan Laboratory, Engineering Research Center of Green Development for Conventional Aquatic Biological Industry in the Yangtze River Economic Belt, Ministry of Education, Huazhong Agricultural University, Wuhan, China.
Organizational Affiliation: