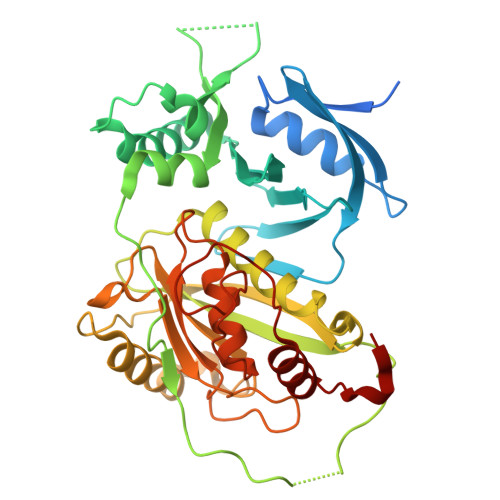
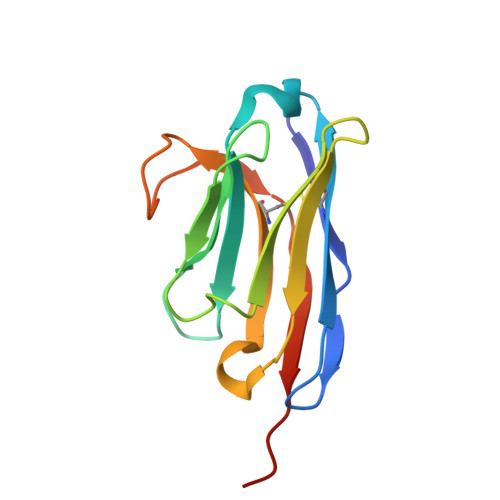
Screening and epitope characterization of diagnostic nanobody against total and activated Bacteroides fragilis toxin.
Guo, Y., Ouyang, Z., He, W., Zhang, J., Qin, Q., Jiao, M., Muyldermans, S., Zheng, F., Wen, Y.(2023) Front Immunol 14: 1065274-1065274
- PubMed: 36845160
- DOI: https://doi.org/10.3389/fimmu.2023.1065274
- Primary Citation of Related Structures:
8H3X, 8H3Y - PubMed Abstract:
Enterotoxigenic Bacteroides fragilis (ETBF) can rapidly secrete an enterotoxin termed B. fragilis toxin (BFT), which is thought to be the only recognized virulence factor in ETBF. ETBF can cause acute diarrhea, inflammatory bowel disease (IBD), colorectal cancer, and breast cancer. BFT is divided into three subtypes, BFT1, BFT2, and BFT3. BFT1 is the most widely distributed in human B. fragilis isolates. BFT can be used as a biomarker for predicting the inflammation-cancer transformation of intestine and breast. Nanobodies have the advantages of small structure, complete antigen recognition capacity, rapid selection via phage display technology, and can be massively produced in microbial expression systems. Nanobodies have become a powerful tool for medical diagnosis and treatment. This study focuses on screening and structural characterization of nanobodies targeting full length and active BFT. By constructing prokaryotic expression systems to obtain recombinant BFT1 protein, high purity BFT1 protein was used to immunize alpacas. Phage display technology was used to construct a phage display library. The positive clones were selected by bio-panning, and the isothermal titration calorimetry was used to select high-affinity nanobodies. Then the three-dimensional structures of BFT1:Nb2.82 and BFT1:Nb3.27 were solved by crystal X-ray diffraction. We got two kinds of nanobodies, Nb2.82 targeting the BFT1 prodomain and Nb3.27 recognizing the BFT1 catalytic domain. This study provides a new strategy for the early diagnosis of ETBF and the possibility for BFT as a biomarker for diagnosing diseases.
- Center for Microbiome Research of Med-X Institute, The Key Laboratory of Environment and Genes Related to Disease of Ministry of Education, The First Affiliated Hospital, Xi'an Jiaotong University, Xi'an, China.
Organizational Affiliation: