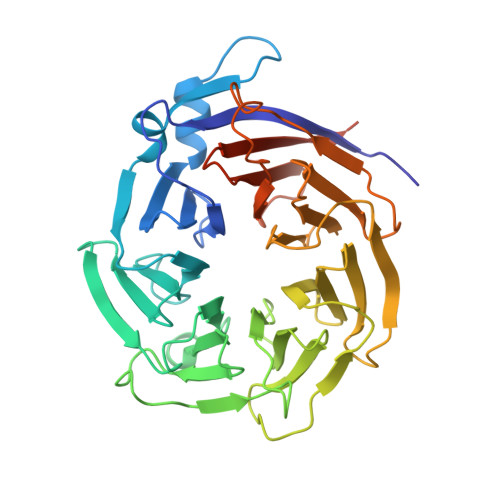
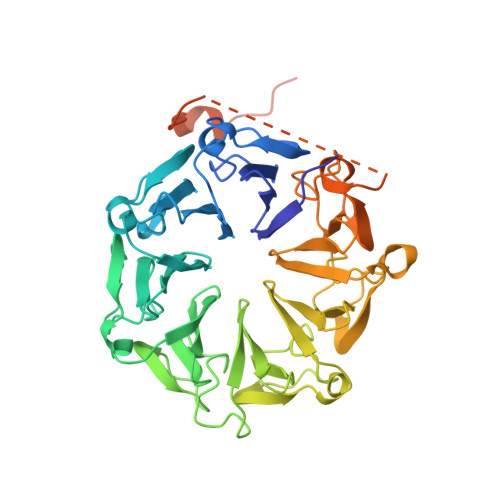
RUP2 facilitates UVR8 redimerization via two interfaces.
Wang, L., Wang, Y., Chang, H., Ren, H., Wu, X., Wen, J., Guan, Z., Ma, L., Qiu, L., Yan, J., Zhang, D., Huang, X., Yin, P.(2023) Plant Commun 4: 100428-100428
- PubMed: 36065466
- DOI: https://doi.org/10.1016/j.xplc.2022.100428
- Primary Citation of Related Structures:
8GQE - PubMed Abstract:
The plant UV-B photoreceptor UV RESISTANCE LOCUS 8 (UVR8) exists as a homodimer in its inactive ground state. Upon UV-B exposure, UVR8 monomerizes and interacts with a downstream key regulator, the CONSTITUTIVE PHOTOMORPHOGENIC 1/SUPPRESSOR OF PHYA (COP1/SPA) E3 ubiquitin ligase complex, to initiate UV-B signaling. Two WD40 proteins, REPRESSOR OF UV-B PHOTOMORPHOGENESIS 1 (RUP1) and RUP2 directly interact with monomeric UVR8 and facilitate UVR8 ground state reversion, completing the UVR8 photocycle. Here, we reconstituted the RUP-mediated UVR8 redimerization process in vitro and reported the structure of the RUP2-UVR8 W285A complex (2.0 Å). RUP2 and UVR8 W285A formed a heterodimer via two distinct interfaces, designated Interface 1 and 2. The previously characterized Interface 1 is found between the RUP2 WD40 domain and the UVR8 C27 subregion. The newly identified Interface 2 is formed through interactions between the RUP2 WD40 domain and the UVR8 core domain. Disruption of Interface 2 impaired UV-B induced photomorphogenic development in Arabidopsis thaliana. Further biochemical analysis indicated that both interfaces are important for RUP2-UVR8 interactions and RUP2-mediated facilitation of UVR8 redimerization. Our findings suggest that the two-interface-interaction mode is adopted by both RUP2 and COP1 when they interact with UVR8, marking a step forward in understanding the molecular basis that underpins the interactions between UVR8 and its photocycle regulators.
- National Key Laboratory of Crop Genetic Improvement, Hubei Hongshan Laboratory, Huazhong Agricultural University, Wuhan 430070, China.
Organizational Affiliation: