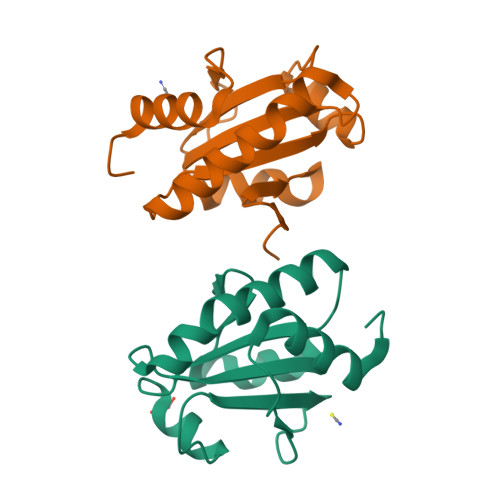
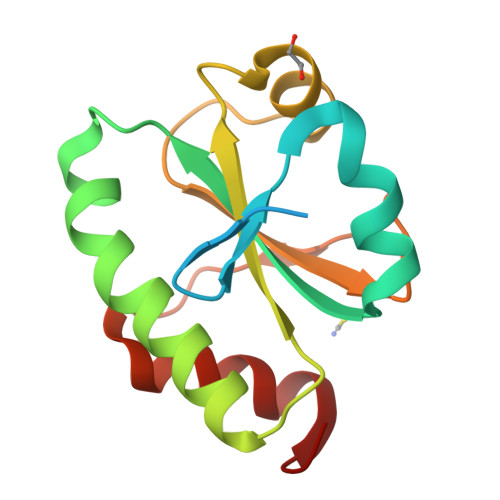
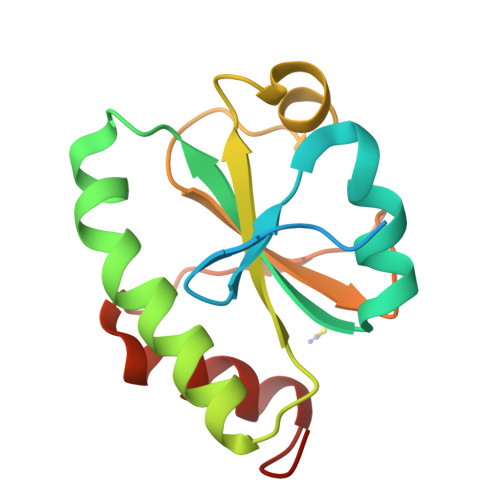
Systematic enhancement of protein crystallization efficiency by bulk lysine-to-arginine (KR) substitution.
Banayan, N.E., Loughlin, B.J., Singh, S., Forouhar, F., Lu, G., Wong, K.H., Neky, M., Hunt, H.S., Bateman Jr., L.B., Tamez, A., Handelman, S.K., Price, W.N., Hunt, J.F.(2024) Protein Sci 33: e4898-e4898
- PubMed: 38358135
- DOI: https://doi.org/10.1002/pro.4898
- Primary Citation of Related Structures:
8GDU, 8GDY - PubMed Abstract:
Structural genomics consortia established that protein crystallization is the primary obstacle to structure determination using x-ray crystallography. We previously demonstrated that crystallization propensity is systematically related to primary sequence, and we subsequently performed computational analyses showing that arginine is the most overrepresented amino acid in crystal-packing interfaces in the Protein Data Bank. Given the similar physicochemical characteristics of arginine and lysine, we hypothesized that multiple lysine-to-arginine (KR) substitutions should improve crystallization. To test this hypothesis, we developed software that ranks lysine sites in a target protein based on the redundancy-corrected KR substitution frequency in homologs. This software can be run interactively on the worldwide web at https://www.pxengineering.org/. We demonstrate that three unrelated single-domain proteins can tolerate 5-11 KR substitutions with at most minor destabilization, and, for two of these three proteins, the construct with the largest number of KR substitutions exhibits significantly enhanced crystallization propensity. This approach rapidly produced a 1.9 Å crystal structure of a human protein domain refractory to crystallization with its native sequence. Structures from Bulk KR-substituted domains show the engineered arginine residues frequently make hydrogen-bonds across crystal-packing interfaces. We thus demonstrate that Bulk KR substitution represents a rational and efficient method for probabilistic engineering of protein surface properties to improve crystallization.
Organizational Affiliation:
Department of Biological Sciences, 702A Sherman Fairchild Center, MC2434, Columbia University, New York, New York, USA.