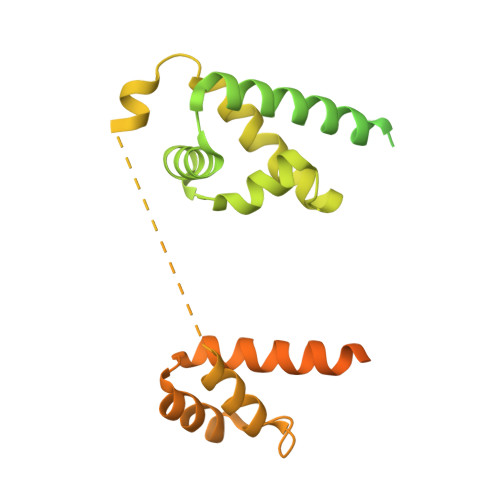
Histone modifications regulate pioneer transcription factor cooperativity.
Sinha, K.K., Bilokapic, S., Du, Y., Malik, D., Halic, M.(2023) Nature 619: 378-384
- PubMed: 37225990
- DOI: https://doi.org/10.1038/s41586-023-06112-6
- Primary Citation of Related Structures:
8G86, 8G87, 8G88, 8G8B, 8G8E, 8G8G - PubMed Abstract:
Pioneer transcription factors have the ability to access DNA in compacted chromatin 1 . Multiple transcription factors can bind together to a regulatory element in a cooperative way, and cooperation between the pioneer transcription factors OCT4 (also known as POU5F1) and SOX2 is important for pluripotency and reprogramming 2-4 . However, the molecular mechanisms by which pioneer transcription factors function and cooperate on chromatin remain unclear. Here we present cryo-electron microscopy structures of human OCT4 bound to a nucleosome containing human LIN28B or nMATN1 DNA sequences, both of which bear multiple binding sites for OCT4. Our structural and biochemistry data reveal that binding of OCT4 induces changes to the nucleosome structure, repositions the nucleosomal DNA and facilitates cooperative binding of additional OCT4 and of SOX2 to their internal binding sites. The flexible activation domain of OCT4 contacts the N-terminal tail of histone H4, altering its conformation and thus promoting chromatin decompaction. Moreover, the DNA-binding domain of OCT4 engages with the N-terminal tail of histone H3, and post-translational modifications at H3K27 modulate DNA positioning and affect transcription factor cooperativity. Thus, our findings suggest that the epigenetic landscape could regulate OCT4 activity to ensure proper cell programming.
- Department of Structural Biology, St. Jude Children's Research Hospital, Memphis, TN, USA.
Organizational Affiliation: