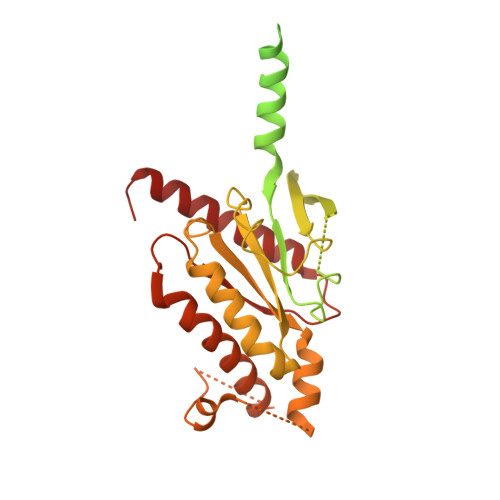
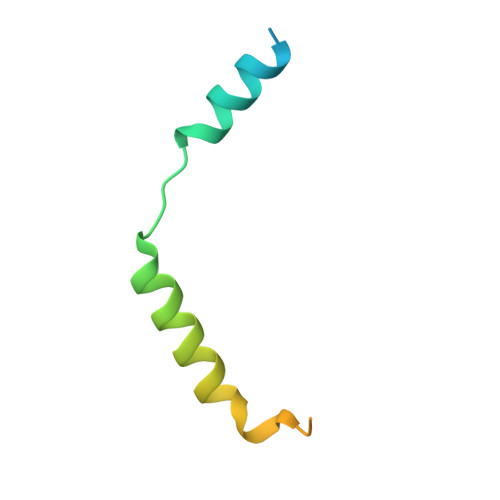
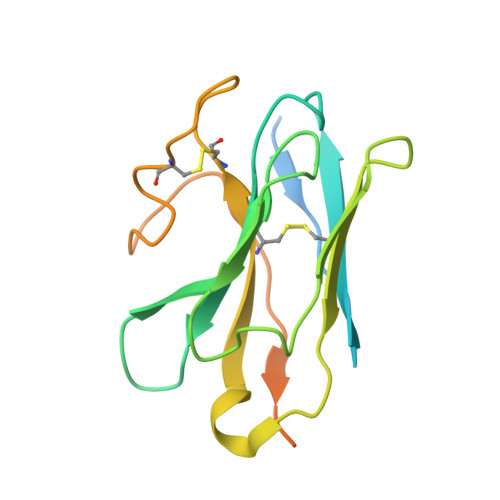
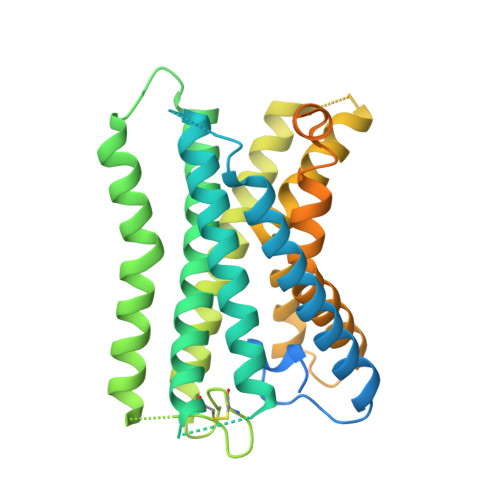
Tethered agonist activated ADGRF1 structure and signalling analysis reveal basis for G protein coupling.
Jones, D.T.D., Dates, A.N., Rawson, S.D., Burruss, M.M., Lipper, C.H., Blacklow, S.C.(2023) Nat Commun 14: 2490-2490
- PubMed: 37120430
- DOI: https://doi.org/10.1038/s41467-023-38083-7
- Primary Citation of Related Structures:
8G2Y - PubMed Abstract:
Adhesion G Protein Coupled Receptors (aGPCRs) have evolved an activation mechanism to translate extracellular force into liberation of a tethered agonist (TA) to effect cell signalling. We report here that ADGRF1 can signal through all major G protein classes and identify the structural basis for a previously reported Gα q preference by cryo-EM. Our structure shows that Gα q preference in ADGRF1 may derive from tighter packing at the conserved F569 of the TA, altering contacts between TM helix I and VII, with a concurrent rearrangement of TM helix VII and helix VIII at the site of Gα recruitment. Mutational studies of the interface and of contact residues within the 7TM domain identify residues critical for signalling, and suggest that Gα s signalling is more sensitive to mutation of TA or binding site residues than Gα q . Our work advances the detailed molecular understanding of aGPCR TA activation, identifying features that potentially explain preferential signal modulation.
- Department of Biological Chemistry and Molecular Pharmacology, Blavatnik Institute, Harvard Medical School, Boston, MA, 02115, USA. daniel_jones@hms.harvard.edu.
Organizational Affiliation: