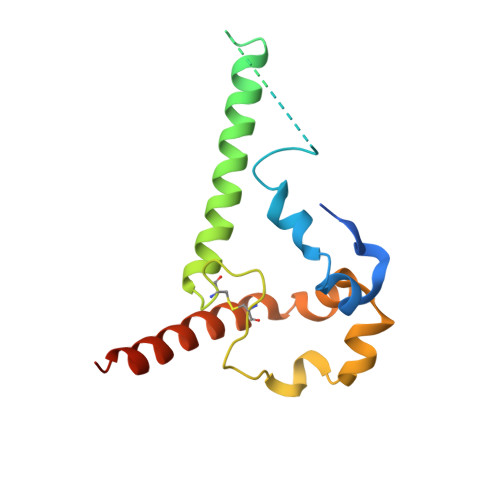
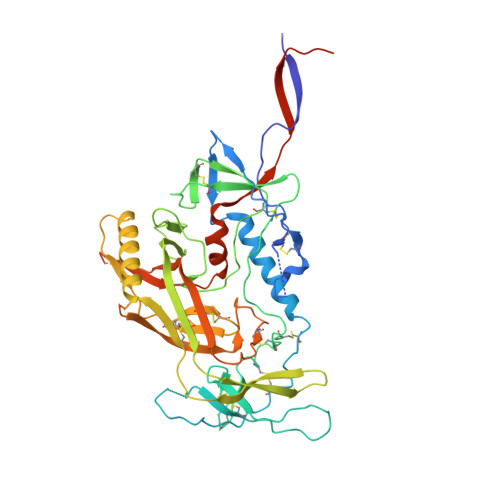
Soluble prefusion-closed HIV-envelope trimers with glycan-covered bases.
Olia, A.S., Cheng, C., Zhou, T., Biju, A., Harris, D.R., Changela, A., Duan, H., Ivleva, V.B., Kong, W.P., Ou, L., Rawi, R., Tsybovsky, Y., Van Wazer, D.J., Corrigan, A.R., Gonelli, C.A., Lee, M., McKee, K., Narpala, S., O'Dell, S., Parchment, D.K., Stancofski, E.D., Stephens, T., Tan, I., Teng, I.T., Wang, S., Wei, Q., Yang, Y., Yang, Z., Zhang, B., Novak, J., Renfrow, M.B., Doria-Rose, N.A., Koup, R.A., McDermott, A.B., Gall, J.G., Lei, Q.P., Mascola, J.R., Kwong, P.D.(2023) iScience 26: 107403-107403
- PubMed: 37554450
- DOI: https://doi.org/10.1016/j.isci.2023.107403
- Primary Citation of Related Structures:
8F7T - PubMed Abstract:
Soluble HIV-1-envelope (Env) trimers elicit immune responses that target their solvent-exposed protein bases, the result of removing these trimers from their native membrane-bound context. To assess whether glycosylation could limit these base responses, we introduced sequons encoding potential N -linked glycosylation sites (PNGSs) into base-proximal regions. Expression and antigenic analyses indicated trimers bearing six-introduced PNGSs to have reduced base recognition. Cryo-EM analysis revealed trimers with introduced PNGSs to be prone to disassembly and introduced PNGS to be disordered. Protein-base and glycan-base trimers induced reciprocally symmetric ELISA responses, in which only a small fraction of the antibody response to glycan-base trimers recognized protein-base trimers and vice versa. EM polyclonal epitope mapping revealed glycan-base trimers -even those that were stable biochemically- to elicit antibodies that recognized disassembled trimers. Introduced glycans can thus mask the protein base but their introduction may yield neo-epitopes that dominate the immune response.
- Vaccine Research Center, National Institutes of Health, Bethesda, MD 20892, USA.
Organizational Affiliation: