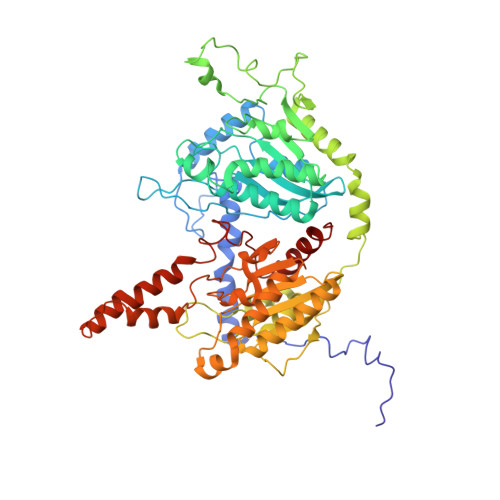
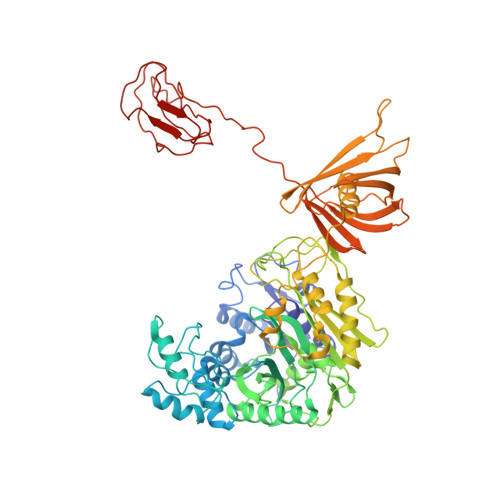
Discovery, structure, and function of filamentous 3-methylcrotonyl-CoA carboxylase.
Hu, J.J., Lee, J.K.J., Liu, Y.T., Yu, C., Huang, L., Aphasizheva, I., Aphasizhev, R., Zhou, Z.H.(2023) Structure 31: 100-110.e4
- PubMed: 36543169
- DOI: https://doi.org/10.1016/j.str.2022.11.015
- Primary Citation of Related Structures:
8F3D, 8F41 - PubMed Abstract:
3-methylcrotonyl-CoA carboxylase (MCC) is a biotin-dependent mitochondrial enzyme necessary for leucine catabolism in most organisms. While the crystal structure of recombinant bacterial MCC has been characterized, the structure and potential polymerization of native MCC remain elusive. Here, we discovered that native MCC from Leishmania tarentolae (LtMCC) forms filaments, and determined the structures of different filament regions at 3.4, 3.9, and 7.3 Å resolution using cryoEM. α 6 β 6 LtMCCs assemble in a twisted-stacks architecture, manifesting as supramolecular rods up to 400 nm. Filamentous LtMCCs bind biotin non-covalently and lack coenzyme A. Filaments elongate by stacking α 6 β 6 LtMCCs onto the exterior α-trimer of the terminal LtMCC. This stacking immobilizes the biotin carboxylase domains, sequestering the enzyme in an inactive state. Our results support a new model for LtMCC catalysis, termed the dual-swinging-domains model, and cast new light on the function of polymerization in the carboxylase superfamily and beyond.
- Department of Microbiology, Immunology, and Molecular Genetics, University of California, Los Angeles (UCLA), Los Angeles, CA 90095, USA; California NanoSystems Institute, UCLA, Los Angeles, CA 90095, USA; Department of Mathematics, UCLA, Los Angeles, CA 90095, USA.
Organizational Affiliation: