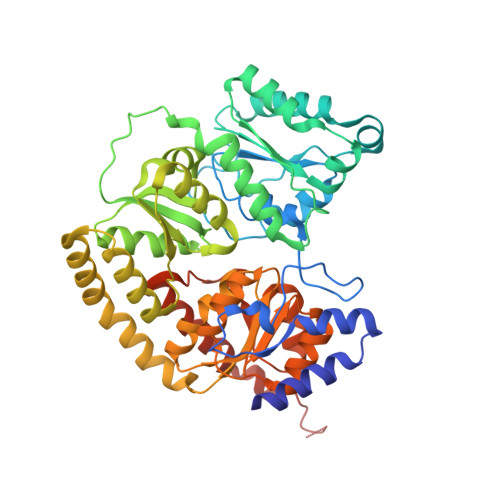
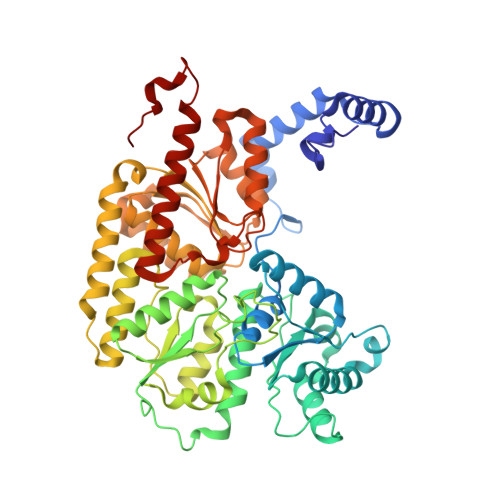
Structural consequences of turnover-induced homocitrate loss in nitrogenase.
Warmack, R.A., Maggiolo, A.O., Orta, A., Wenke, B.B., Howard, J.B., Rees, D.C.(2023) Nat Commun 14: 1091-1091
- PubMed: 36841829
- DOI: https://doi.org/10.1038/s41467-023-36636-4
- Primary Citation of Related Structures:
8CRS, 8DBX, 8ENL, 8ENM, 8ENN, 8ENO - PubMed Abstract:
Nitrogenase catalyzes the ATP-dependent reduction of dinitrogen to ammonia during the process of biological nitrogen fixation that is essential for sustaining life. The active site FeMo-cofactor contains a [7Fe:1Mo:9S:1C] metallocluster coordinated with an R-homocitrate (HCA) molecule. Here, we establish through single particle cryoEM and chemical analysis of two forms of the Azotobacter vinelandii MoFe-protein - a high pH turnover inactivated species and a ∆NifV variant that cannot synthesize HCA - that loss of HCA is coupled to α-subunit domain and FeMo-cofactor disordering, and formation of a histidine coordination site. We further find a population of the ∆NifV variant complexed to an endogenous protein identified through structural and proteomic approaches as the uncharacterized protein NafT. Recognition by endogenous NafT demonstrates the physiological relevance of the HCA-compromised form, perhaps for cofactor insertion or repair. Our results point towards a dynamic active site in which HCA plays a role in enabling nitrogenase catalysis by facilitating activation of the FeMo-cofactor from a relatively stable form to a state capable of reducing dinitrogen under ambient conditions.
- Division of Chemistry and Chemical Engineering 147-75, California Institute of Technology, Pasadena, CA, 91125, USA. rwarmack@caltech.edu.
Organizational Affiliation: