Mechanism of 4-aminopyridine inhibition of the lysosomal channel TMEM175.
Oh, S., Stix, R., Zhou, W., Faraldo-Gomez, J.D., Hite, R.K.(2022) Proc Natl Acad Sci U S A 119: e2208882119-e2208882119
- PubMed: 36279431
- DOI: https://doi.org/10.1073/pnas.2208882119
- Primary Citation of Related Structures:
8DHM - PubMed Abstract:
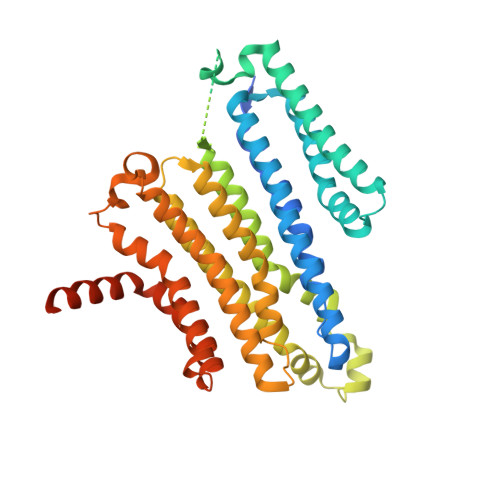
Transmembrane protein 175 (TMEM175) is an evolutionarily distinct lysosomal cation channel whose mutation is associated with the development of Parkinson's disease. Here, we present a cryoelectron microscopy structure and molecular simulations of TMEM175 bound to 4-aminopyridine (4-AP), the only known small-molecule inhibitor of TMEM175 and a broad K + channel inhibitor, as well as a drug approved by the Food and Drug Administration against multiple sclerosis. The structure shows that 4-AP, whose mode of action had not been previously visualized, binds near the center of the ion conduction pathway, in the open state of the channel. Molecular dynamics simulations reveal that this binding site is near the middle of the transmembrane potential gradient, providing a rationale for the voltage-dependent dissociation of 4-AP from TMEM175. Interestingly, bound 4-AP rapidly switches between three predominant binding poses, stabilized by alternate interaction patterns dictated by the twofold symmetry of the channel. Despite this highly dynamic binding mode, bound 4-AP prevents not only ion permeation but also water flow. Together, these studies provide a framework for the rational design of novel small-molecule inhibitors of TMEM175 that might reveal the role of this channel in human lysosomal physiology both in health and disease.
- Structural Biology Program, Memorial Sloan Kettering Cancer Center, New York, NY 10065.
Organizational Affiliation: