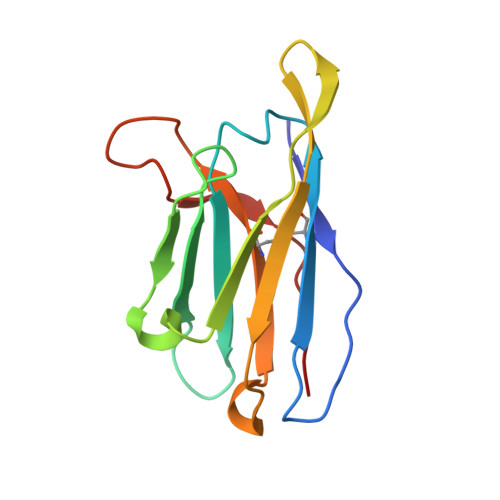
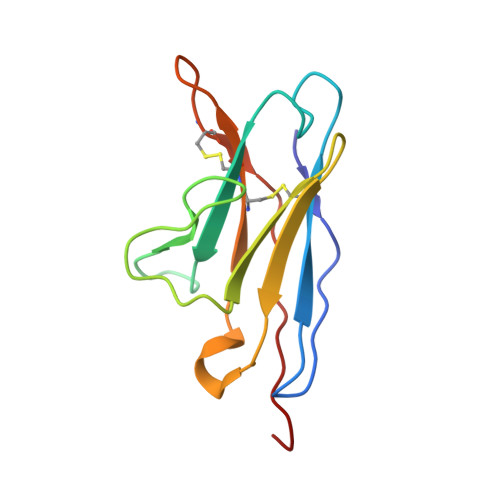
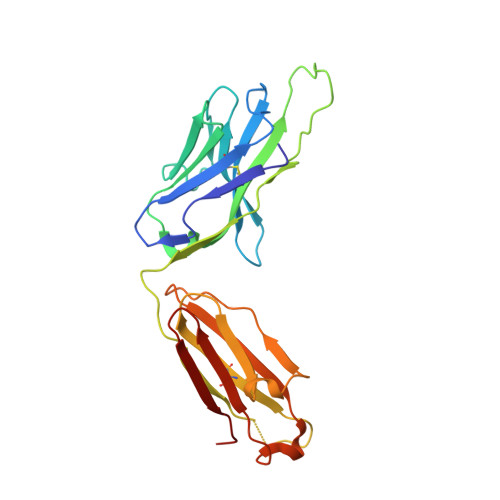
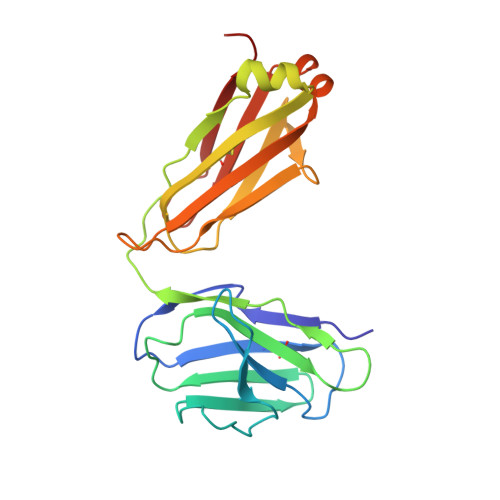
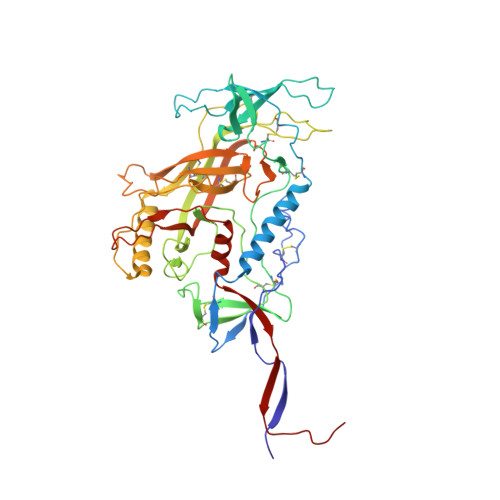
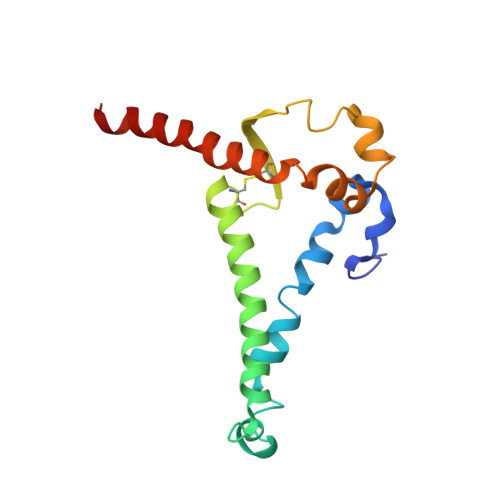
Germline-targeting HIV vaccination induces neutralizing antibodies to the CD4 binding site.
Caniels, T.G., Medina-Ramirez, M., Zhang, S., Kratochvil, S., Xian, Y., Koo, J.H., Derking, R., Samsel, J., van Schooten, J., Pecetta, S., Lamperti, E., Yuan, M., Carrasco, M.R., Del Moral Sanchez, I., Allen, J.D., Bouhuijs, J.H., Yasmeen, A., Ketas, T.J., Snitselaar, J.L., Bijl, T.P.L., Martin, I.C., Torres, J.L., Cupo, A., Shirreff, L., Rogers, K., Mason, R.D., Roederer, M., Greene, K.M., Gao, H., Silva, C.M., Baken, I.J.L., Tian, M., Alt, F.W., Pulendran, B., Seaman, M.S., Crispin, M., van Gils, M.J., Montefiori, D.C., McDermott, A.B., Villinger, F.J., Koup, R.A., Moore, J.P., Klasse, P.J., Ozorowski, G., Batista, F.D., Wilson, I.A., Ward, A.B., Sanders, R.W.(2024) Sci Immunol 9: eadk9550-eadk9550
- PubMed: 39213338
- DOI: https://doi.org/10.1126/sciimmunol.adk9550
- Primary Citation of Related Structures:
8D01, 8D0Y, 8SW3, 8SW4 - PubMed Abstract:
Eliciting potent and broadly neutralizing antibodies (bnAbs) is a major goal in HIV-1 vaccine development. Here, we describe how germline-targeting immunogen BG505 SOSIP germline trimer 1.1 (GT1.1), generated through structure-based design, engages a diverse range of VRC01-class bnAb precursors. A single immunization with GT1.1 expands CD4 binding site (CD4bs)-specific VRC01-class B cells in knock-in mice and drives VRC01-class maturation. In nonhuman primates (NHPs), GT1.1 primes CD4bs-specific neutralizing serum responses. Selected monoclonal antibodies (mAbs) isolated from GT1.1-immunized NHPs neutralize fully glycosylated BG505 virus. Two mAbs, 12C11 and 21N13, neutralize subsets of diverse heterologous neutralization-resistant viruses. High-resolution structures revealed that 21N13 targets the same conserved residues in the CD4bs as VRC01-class and CH235-class bnAbs despite its low sequence similarity (~40%), whereas mAb 12C11 binds predominantly through its heavy chain complementarity-determining region 3. These preclinical data underpin the ongoing evaluation of GT1.1 in a phase 1 clinical trial in healthy volunteers.
- Amsterdam UMC, location AMC, University of Amsterdam, Department of Medical Microbiology and Infection Prevention, Amsterdam, Netherlands.
Organizational Affiliation: