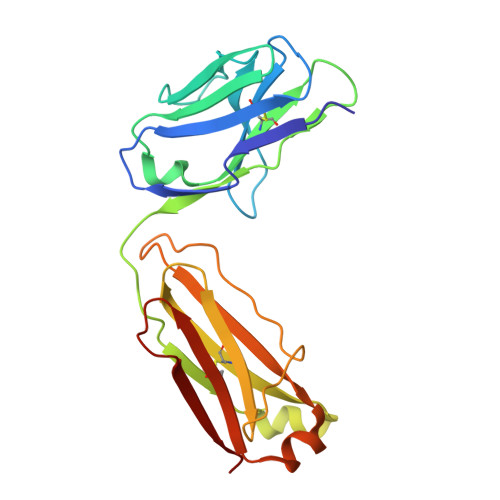
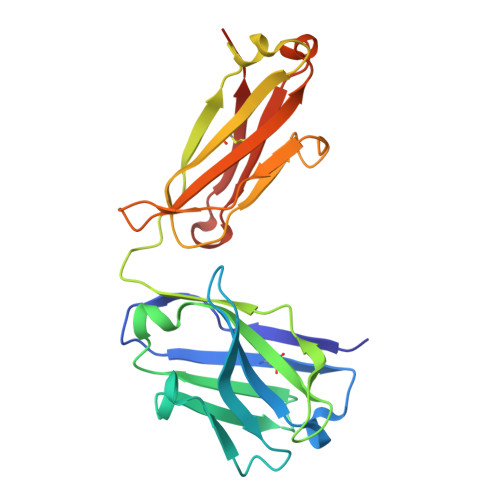
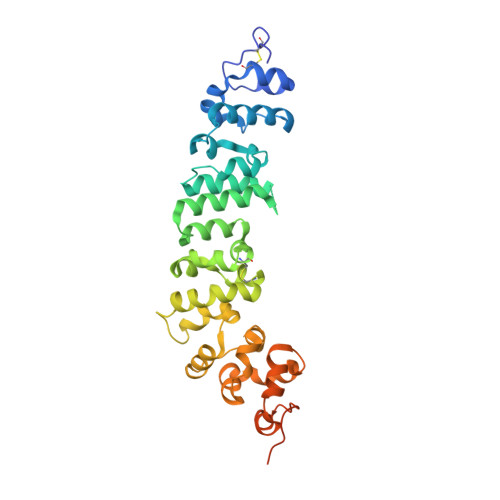
Novel mesothelin antibodies enable crystallography of the intact mesothelin ectodomain and engineering of potent, T cell-engaging bispecific therapeutics
Lin, I., Rupert, P.B., Pilat, K., Ruff, R.O., Friend, D.J., Chan, M.K., Clarke, M., Hoffstrom, B.G., Carter, J., Meshinchi, S., Bandaranayake, A.D., Mehlin, C., Olson, J.M., Strong, R.K., Correnti, C.E.(2023) Front Drug Discov 3