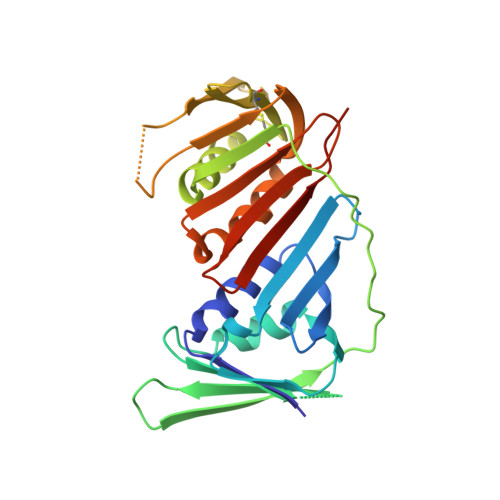
ERCC6L2 mitigates replication stress and promotes centromere stability.
Carnie, C.J., Armstrong, L., Sebesta, M., Ariza, A., Wang, X., Graham, E., Zhu, K., Ahel, D.(2023) Cell Rep 42: 112329-112329
- PubMed: 37014751
- DOI: https://doi.org/10.1016/j.celrep.2023.112329
- Primary Citation of Related Structures:
8COB - PubMed Abstract:
Structurally complex genomic regions, such as centromeres, are inherently difficult to duplicate. The mechanism behind centromere inheritance is not well understood, and one of the key questions relates to the reassembly of centromeric chromatin following DNA replication. Here, we define ERCC6L2 as a key regulator of this process. ERCC6L2 accumulates at centromeres and promotes deposition of core centromeric factors. Interestingly, ERCC6L2 -/- cells show unrestrained replication of centromeric DNA, likely caused by the erosion of centromeric chromatin. Beyond centromeres, ERCC6L2 facilitates replication at genomic repeats and non-canonical DNA structures. Notably, ERCC6L2 interacts with the DNA-clamp PCNA through an atypical peptide, presented here in a co-crystal structure. Finally, ERCC6L2 also restricts DNA end resection, acting independently of the 53BP1-REV7-Shieldin complex. We propose a mechanistic model, which reconciles seemingly distinct functions of ERCC6L2 in DNA repair and DNA replication. These findings provide a molecular context for studies linking ERCC6L2 to human disease.
- Sir William Dunn School of Pathology, University of Oxford, Oxford OX1 3RE, UK.
Organizational Affiliation: