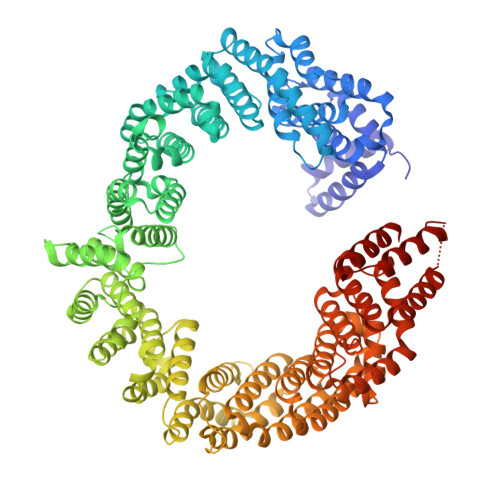
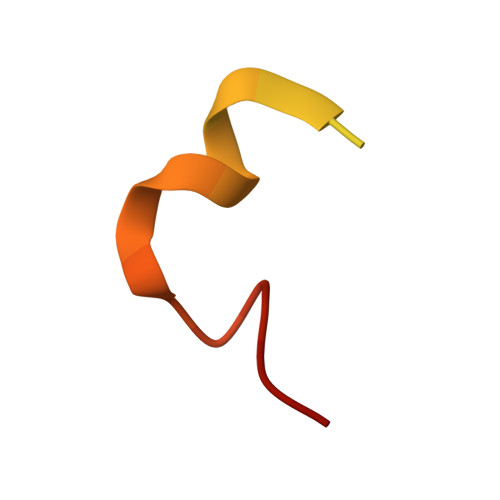
Structural basis of phosphorylation-independent nuclear import of CIRBP by TNPO3.
Zhou, Q., Sagmeister, T., Hutten, S., Bourgeois, B., Pavkov-Keller, T., Dormann, D., Madl, T.(2025) Nat Commun 16: 4456-4456
- PubMed: 40360518
- DOI: https://doi.org/10.1038/s41467-025-59802-2
- Primary Citation of Related Structures:
8CMK - PubMed Abstract:
Transportin 3 (TNPO3) is a nuclear import receptor known for its broad substrate specificity, often recognizing arginine-serine (SR/RS) repeat-rich nuclear localization signals (NLS) in SRSF proteins. While serine phosphorylation or glutamate presence has been associated with these NLSs, recent proteomic studies identified TNPO3 cargoes lacking SR/RS repeats. One such example is the cold-inducible RNA-binding protein (CIRBP), which contains a non-classical RSY-NLS. Using X-ray crystallography, here we investigate the TNPO3-CIRBP interaction and find that tyrosines within the RSY-NLS play a key role in binding, independent of phosphorylation. Surprisingly, serine and tyrosine phosphorylation in CIRBP's NLS inhibits TNPO3 binding, suggesting a regulatory mechanism for nuclear import. Our study reveals a non-conventional nuclear import mechanism mediated by TNPO3, which may extend to other known or yet undiscovered TNPO3 cargoes.
- Research Unit Integrative Structural Biology, Medicinal Chemistry, Otto Loewi Research Center, Medical University of Graz, Graz, Austria.
Organizational Affiliation: