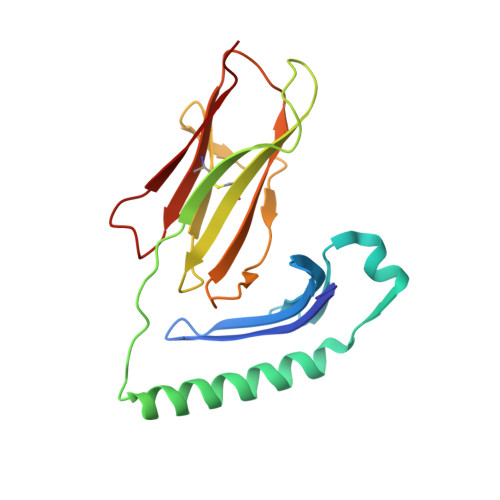
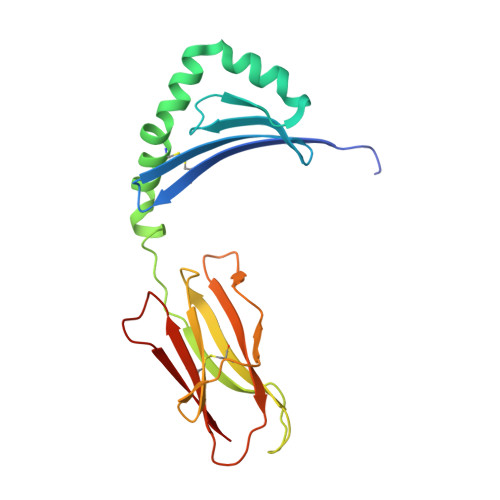
Structural definition of HLA class II-presented SARS-CoV-2 epitopes reveals a mechanism to escape pre-existing CD4 + T cell immunity.
Chen, Y., Mason, G.H., Scourfield, D.O., Greenshields-Watson, A., Haigh, T.A., Sewell, A.K., Long, H.M., Gallimore, A.M., Rizkallah, P., MacLachlan, B.J., Godkin, A.(2023) Cell Rep 42: 112827-112827
- PubMed: 37471227
- DOI: https://doi.org/10.1016/j.celrep.2023.112827
- Primary Citation of Related Structures:
8CMB, 8CMC, 8CMD, 8CME, 8CMF, 8CMG, 8CMH, 8CMI - PubMed Abstract:
CD4 + T cells recognize a broad range of peptide epitopes of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which contribute to immune memory and limit COVID-19 disease. We demonstrate that the immunogenicity of SARS-CoV-2 peptides, in the context of the model allotype HLA-DR1, does not correlate with their binding affinity to the HLA heterodimer. Analyzing six epitopes, some with very low binding affinity, we solve X-ray crystallographic structures of each bound to HLA-DR1. Further structural definitions reveal the precise molecular impact of viral variant mutations on epitope presentation. Omicron escaped ancestral SARS-CoV-2 immunity to two epitopes through two distinct mechanisms: (1) mutations to TCR-facing epitope positions and (2) a mechanism whereby a single amino acid substitution caused a register shift within the HLA binding groove, completely altering the peptide-HLA structure. This HLA-II-specific paradigm of immune escape highlights how CD4 + T cell memory is finely poised at the level of peptide-HLA-II presentation.
- Division of Infection and Immunity, School of Medicine, Cardiff University, Cardiff CF14 4XN, UK; Systems Immunity University Research Institute, School of Medicine, Cardiff University, Cardiff CF14 4XN, UK.
Organizational Affiliation: