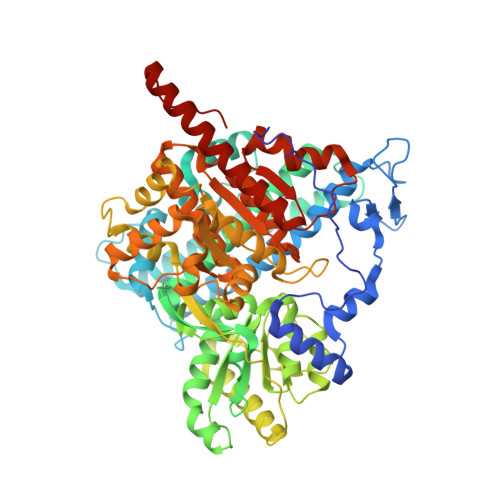
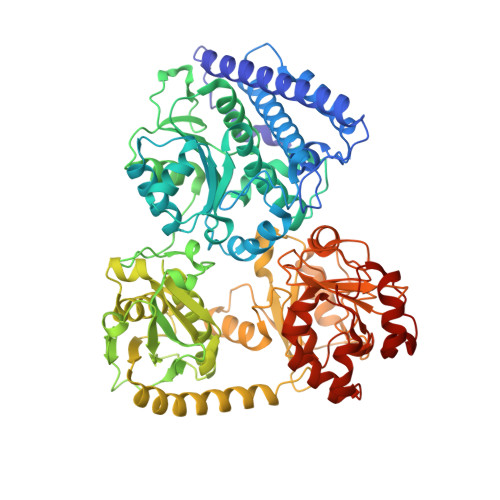
Coupling CO 2 Reduction and Acetyl-CoA Formation: The Role of a CO Capturing Tunnel in Enzymatic Catalysis.
Ruickoldt, J., Jeoung, J.H., Rudolph, M.A., Lennartz, F., Kreibich, J., Schomacker, R., Dobbek, H.(2024) Angew Chem Int Ed Engl 63: e202405120-e202405120
- PubMed: 38743001
- DOI: https://doi.org/10.1002/anie.202405120
- Primary Citation of Related Structures:
7ZKV, 8CJA, 8CJB, 8CJC, 8CMW - PubMed Abstract:
The bifunctional CO-dehydrogenase/acetyl-CoA synthase (CODH/ACS) complex couples the reduction of CO2 to the condensation of CO with a methyl-moiety and CoA to acetyl-CoA. Catalysis occurs at two sites connected by a tunnel transporting the CO. Here, we investigated how the bifunctional complex and its tunnel support catalysis using the CODH/ACS from Carboxydothermus hydrogenoformans as a model. Although CODH/ACS adapted to form a stable bifunctional complex with a secluded substrate tunnel, catalysis and CO transport is even more efficient when two monofunctional enzymes are coupled. Efficient CO channeling appears to be ensured by hydrophobic binding sites for CO, which act in a bucket-brigade fashion rather than as a simple tube. Tunnel remodeling showed that opening the tunnel increased activity but impaired directed transport of CO. Constricting the tunnel impaired activity and CO transport, suggesting that the tunnel evolved to sequester CO rather than to maximize turnover.
- Humboldt-Universität zu Berlin, Biology, GERMANY.
Organizational Affiliation: