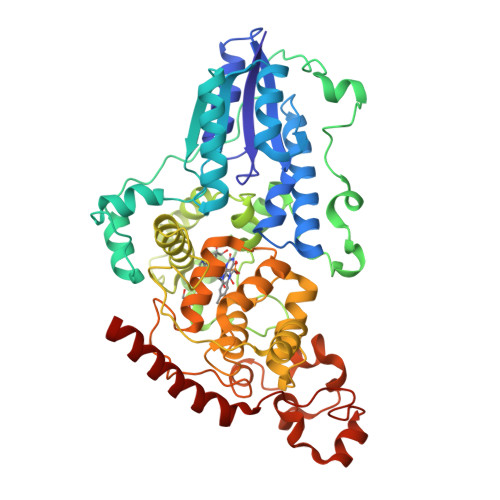
Directed ultrafast conformational changes accompany electron transfer in a photolyase as resolved by serial crystallography.
Cellini, A., Shankar, M.K., Nimmrich, A., Hunt, L.A., Monrroy, L., Mutisya, J., Furrer, A., Beale, E.V., Carrillo, M., Malla, T.N., Maj, P., Vrhovac, L., Dworkowski, F., Cirelli, C., Johnson, P.J.M., Ozerov, D., Stojkovic, E.A., Hammarstrom, L., Bacellar, C., Standfuss, J., Maj, M., Schmidt, M., Weinert, T., Ihalainen, J.A., Wahlgren, W.Y., Westenhoff, S.(2024) Nat Chem 16: 624-632
- PubMed: 38225270
- DOI: https://doi.org/10.1038/s41557-023-01413-9
- Primary Citation of Related Structures:
8C1U, 8C69, 8C6A, 8C6B, 8C6C, 8C6F, 8C6H - PubMed Abstract:
Charge-transfer reactions in proteins are important for life, such as in photolyases which repair DNA, but the role of structural dynamics remains unclear. Here, using femtosecond X-ray crystallography, we report the structural changes that take place while electrons transfer along a chain of four conserved tryptophans in the Drosophila melanogaster (6-4) photolyase. At femto- and picosecond delays, photoreduction of the flavin by the first tryptophan causes directed structural responses at a key asparagine, at a conserved salt bridge, and by rearrangements of nearby water molecules. We detect charge-induced structural changes close to the second tryptophan from 1 ps to 20 ps, identifying a nearby methionine as an active participant in the redox chain, and from 20 ps around the fourth tryptophan. The photolyase undergoes highly directed and carefully timed adaptations of its structure. This questions the validity of the linear solvent response approximation in Marcus theory and indicates that evolution has optimized fast protein fluctuations for optimal charge transfer.
Organizational Affiliation:
Department of Chemistry and Molecular Biology, University of Gothenburg, Gothenburg, Sweden.