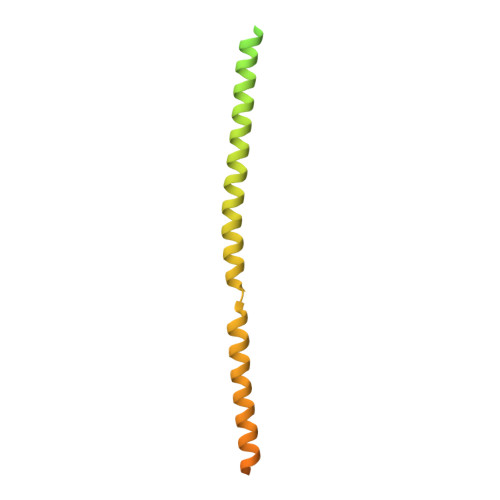Structural and biophysical characterization of the Borna disease virus 1 phosphoprotein.
Whitehead, J.D., Grimes, J.M., Keown, J.R.(2023) Acta Crystallogr F Struct Biol Commun 79: 51-60
- PubMed: 36862093
- DOI: https://doi.org/10.1107/S2053230X23000717
- Primary Citation of Related Structures:
8BS7 - PubMed Abstract:
Bornaviruses are RNA viruses with a mammalian, reptilian, and avian host range. The viruses infect neuronal cells and in rare cases cause a lethal encephalitis. The family Bornaviridae are part of the Mononegavirales order of viruses, which contain a nonsegmented viral genome. Mononegavirales encode a viral phosphoprotein (P) that binds both the viral polymerase (L) and the viral nucleoprotein (N). The P protein acts as a molecular chaperone and is required for the formation of a functional replication/transcription complex. In this study, the structure of the oligomerization domain of the phosphoprotein determined by X-ray crystallography is reported. The structural results are complemented with biophysical characterization using circular dichroism, differential scanning calorimetry and small-angle X-ray scattering. The data reveal the phosphoprotein to assemble into a stable tetramer, with the regions outside the oligomerization domain remaining highly flexible. A helix-breaking motif is observed between the α-helices at the midpoint of the oligomerization domain that appears to be conserved across the Bornaviridae. These data provide information on an important component of the bornavirus replication complex.
- Division of Structural Biology, Wellcome Trust Centre for Human Genetics, University of Oxford, Oxford, United Kingdom.
Organizational Affiliation: