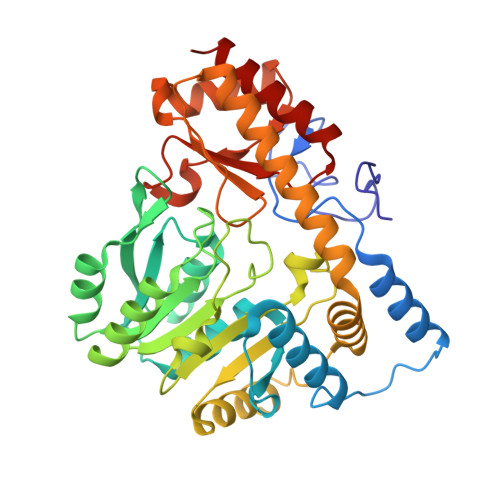
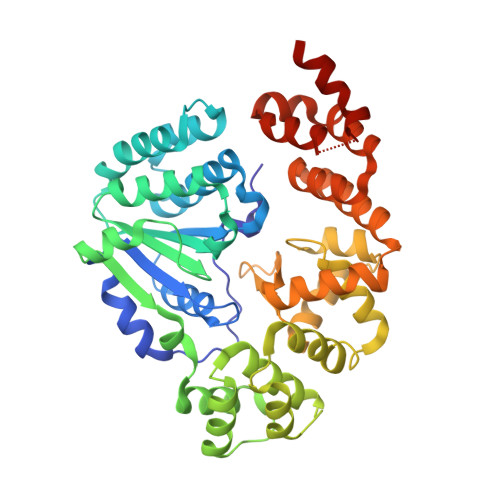
Structural basis for negative regulation of the Escherichia coli maltose system.
Wu, Y., Sun, Y., Richet, E., Han, Z., Chai, J.(2023) Nat Commun 14: 4925-4925
- PubMed: 37582800
- DOI: https://doi.org/10.1038/s41467-023-40447-y
- Primary Citation of Related Structures:
8BOB - PubMed Abstract:
Proteins from the signal transduction ATPases with numerous domains (STAND) family are known to play an important role in innate immunity. However, it remains less well understood how they function in transcriptional regulation. MalT is a bacterial STAND that controls the Escherichia coli maltose system. Inactive MalT is sequestered by different inhibitory proteins such as MalY. Here, we show that MalY interacts with one oligomerization interface of MalT to form a 2:2 complex. MalY represses MalT activity by blocking its oligomerization and strengthening ADP-mediated MalT autoinhibition. A loop region N-terminal to the nucleotide-binding domain (NBD) of MalT has a dual role in mediating MalT autoinhibition and activation. Structural comparison shows that ligand-binding induced oligomerization is required for stabilizing the C-terminal domains and conferring DNA-binding activity. Together, our study reveals the mechanism whereby a prokaryotic STAND is inhibited by a repressor protein and offers insights into signaling by STAND transcription activators.
- Institute of Biochemistry, University of Cologne, Cologne, Germany.
Organizational Affiliation: