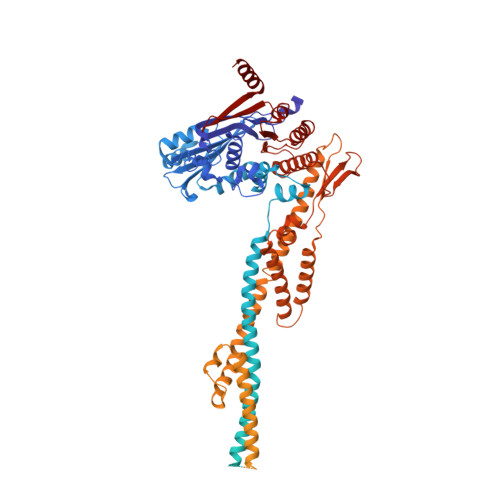
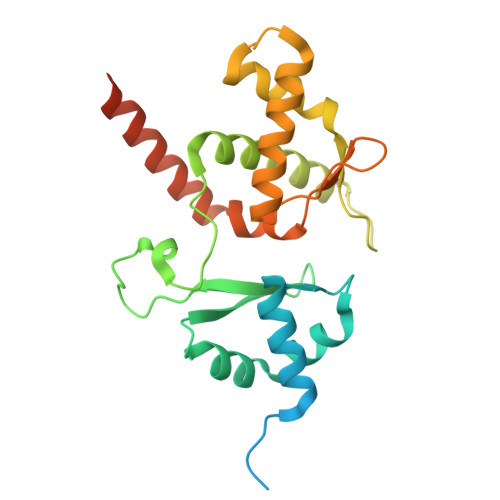
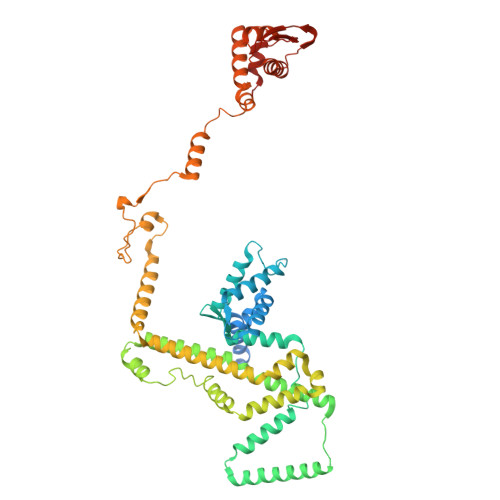
DNA-measuring Wadjet SMC ATPases restrict smaller circular plasmids by DNA cleavage.
Liu, H.W., Roisne-Hamelin, F., Beckert, B., Li, Y., Myasnikov, A., Gruber, S.(2022) Mol Cell 82: 4727-4740.e6
- PubMed: 36525956
- DOI: https://doi.org/10.1016/j.molcel.2022.11.015
- Primary Citation of Related Structures:
8AS8, 8BFN - PubMed Abstract:
Structural maintenance of chromosome (SMC) complexes fold DNA by loop extrusion to support chromosome segregation and genome maintenance. Wadjet systems (JetABCD/MksBEFG/EptABCD) are derivative SMC complexes with roles in bacterial immunity against selfish DNA. Here, we show that JetABCD restricts circular plasmids with an upper size limit of about 100 kb, whereas a linear plasmid evades restriction. Purified JetABCD complexes cleave circular DNA molecules, regardless of the DNA helical topology; cleavage is DNA sequence nonspecific and depends on the SMC ATPase. A cryo-EM structure reveals a distinct JetABC dimer-of-dimers geometry, with the two SMC dimers facing in opposite direction-rather than the same as observed with MukBEF. We hypothesize that JetABCD is a DNA-shape-specific endonuclease and propose the "total extrusion model" for DNA cleavage exclusively when extrusion of an entire plasmid has been completed by a JetABCD complex. Total extrusion cannot be achieved on the larger chromosome, explaining how self-DNA may evade processing.
- Department of Fundamental Microbiology (DMF), Faculty of Biology and Medicine (FBM), University of Lausanne (UNIL), 1015 Lausanne, Switzerland.
Organizational Affiliation: