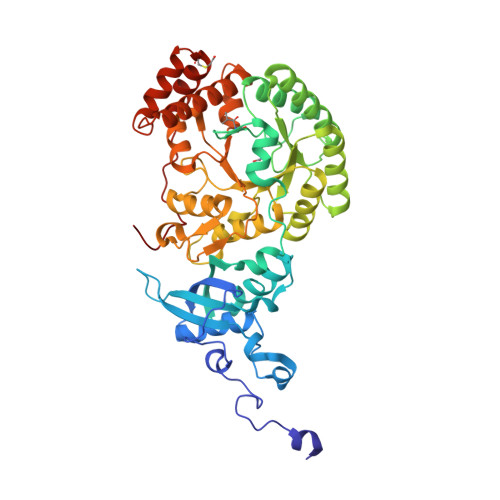
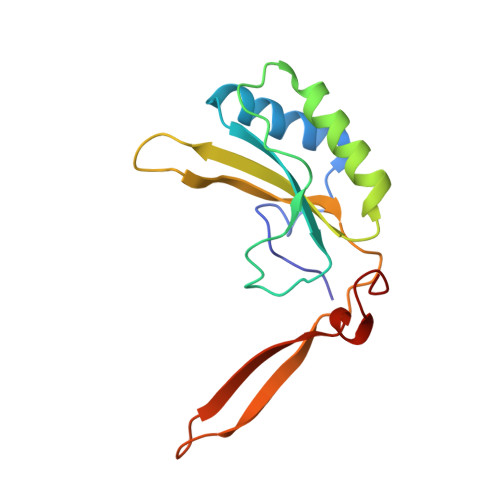
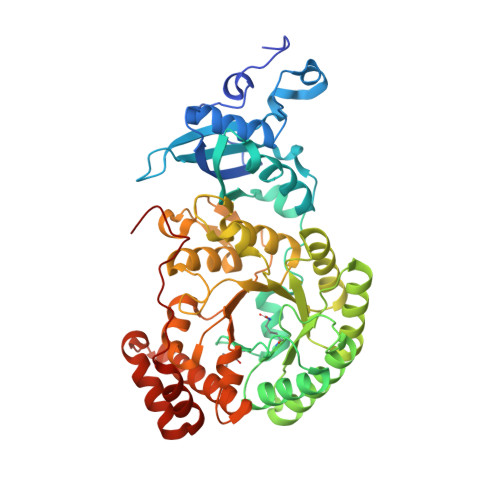
Grafting Rhodobacter sphaeroides with red algae Rubisco to accelerate catalysis and plant growth.
Zhou, Y., Gunn, L.H., Birch, R., Andersson, I., Whitney, S.M.(2023) Nat Plants 9: 978-986
- PubMed: 37291398
- DOI: https://doi.org/10.1038/s41477-023-01436-7
- Primary Citation of Related Structures:
8BDB - PubMed Abstract:
Improving the carboxylation properties of Rubisco has primarily arisen from unforeseen amino acid substitutions remote from the catalytic site. The unpredictability has frustrated rational design efforts to enhance plant Rubisco towards the prized growth-enhancing carboxylation properties of red algae Griffithsia monilis GmRubisco. To address this, we determined the crystal structure of GmRubisco to 1.7 Å. Three structurally divergent domains were identified relative to the red-type bacterial Rhodobacter sphaeroides RsRubisco that, unlike GmRubisco, are expressed in Escherichia coli and plants. Kinetic comparison of 11 RsRubisco chimaeras revealed that incorporating C329A and A332V substitutions from GmRubisco Loop 6 (corresponding to plant residues 328 and 331) into RsRubisco increased the carboxylation rate (k cat c ) by 60%, the carboxylation efficiency in air by 22% and the CO 2 /O 2 specificity (S c/o ) by 7%. Plastome transformation of this RsRubisco Loop 6 mutant into tobacco enhanced photosynthesis and growth up to twofold over tobacco producing wild-type RsRubisco. Our findings demonstrate the utility of RsRubisco for the identification and in planta testing of amino acid grafts from algal Rubisco that can enhance the enzyme's carboxylase potential.
- Plant Science Division, Research School of Biology, The Australian National University, Canberra, Australian Capital Territory, Australia.
Organizational Affiliation: