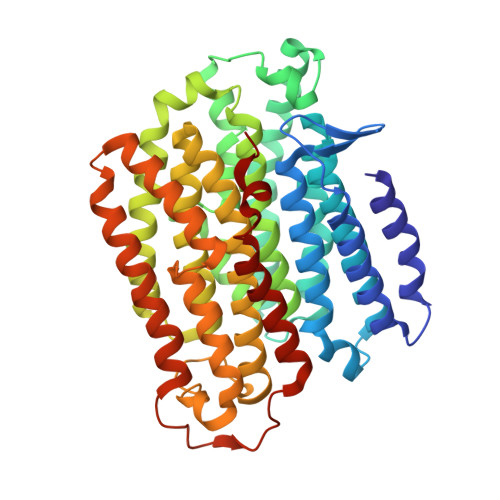
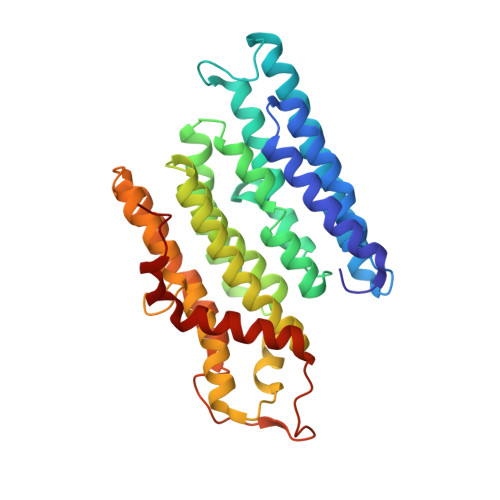
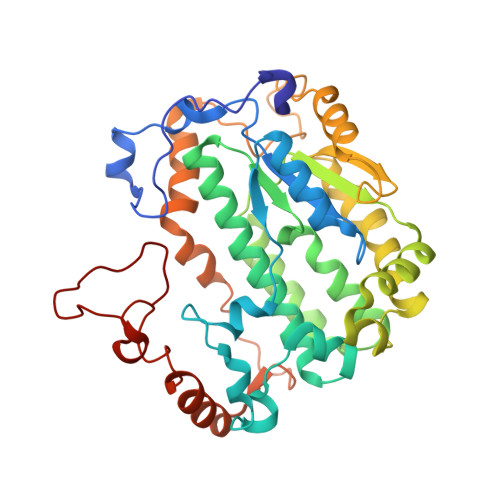
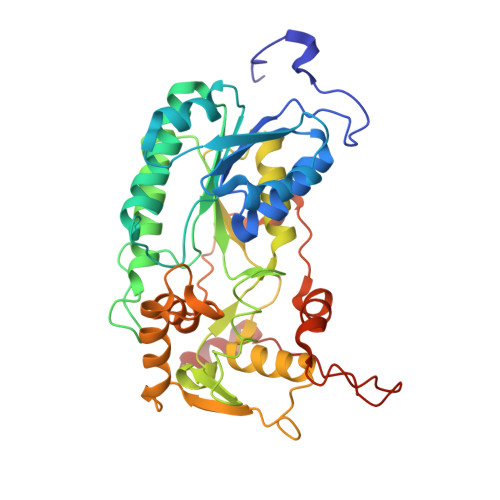
Cryo-EM structures of mitochondrial respiratory complex I from Drosophila melanogaster.
Agip, A.A., Chung, I., Sanchez-Martinez, A., Whitworth, A.J., Hirst, J.(2023) Elife 12
- PubMed: 36622099
- DOI: https://doi.org/10.7554/eLife.84424
- Primary Citation of Related Structures:
8B9Z, 8BA0 - PubMed Abstract:
Respiratory complex I powers ATP synthesis by oxidative phosphorylation, exploiting the energy from NADH oxidation by ubiquinone to drive protons across an energy-transducing membrane. Drosophila melanogaster is a candidate model organism for complex I due to its high evolutionary conservation with the mammalian enzyme, well-developed genetic toolkit, and complex physiology for studies in specific cell types and tissues. Here, we isolate complex I from Drosophila and determine its structure, revealing a 43-subunit assembly with high structural homology to its 45-subunit mammalian counterpart, including a hitherto unknown homologue to subunit NDUFA3. The major conformational state of the Drosophila enzyme is the mammalian-type 'ready-to-go' active resting state, with a fully ordered and enclosed ubiquinone-binding site, but a subtly altered global conformation related to changes in subunit ND6. The mammalian-type 'deactive' pronounced resting state is not observed: in two minor states, the ubiquinone-binding site is unchanged, but a deactive-type π-bulge is present in ND6-TMH3. Our detailed structural knowledge of Drosophila complex I provides a foundation for new approaches to disentangle mechanisms of complex I catalysis and regulation in bioenergetics and physiology.
- The Medical Research Council Mitochondrial Biology Unit, University of Cambridge, The Keith Peters Building, Cambridge Biomedical Campus, Cambridge, United Kingdom.
Organizational Affiliation: