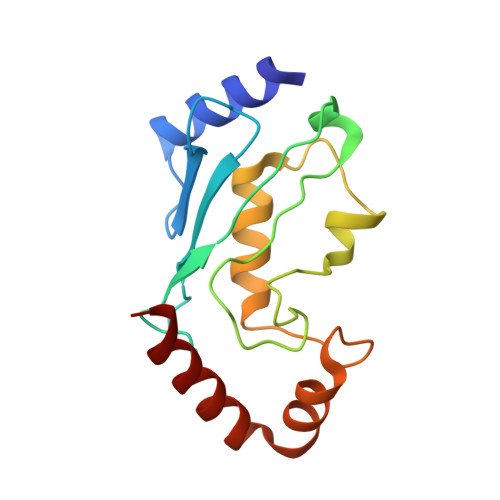
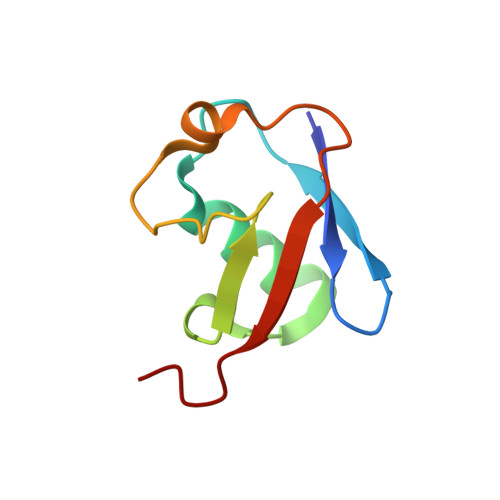
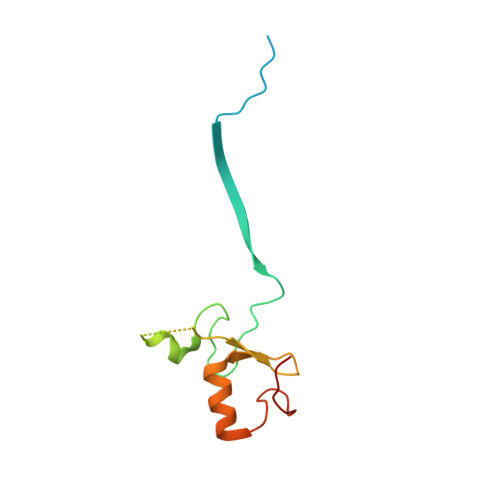
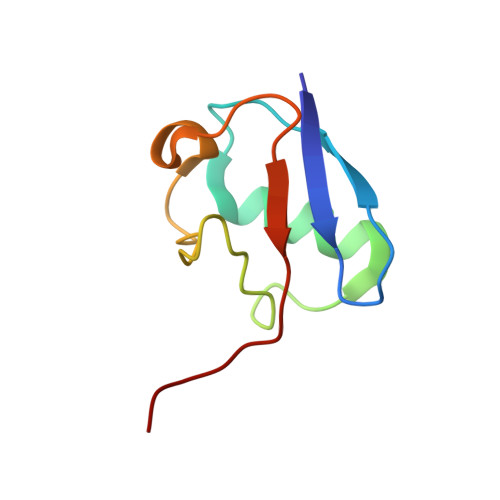
Structural basis for RNA polymerase II ubiquitylation and inactivation in transcription-coupled repair.
Kokic, G., Yakoub, G., van den Heuvel, D., Wondergem, A.P., van der Meer, P.J., van der Weegen, Y., Chernev, A., Fianu, I., Fokkens, T.J., Lorenz, S., Urlaub, H., Cramer, P., Luijsterburg, M.S.(2024) Nat Struct Mol Biol 31: 536-547
- PubMed: 38316879
- DOI: https://doi.org/10.1038/s41594-023-01207-0
- Primary Citation of Related Structures:
8B3G - PubMed Abstract:
During transcription-coupled DNA repair (TCR), RNA polymerase II (Pol II) transitions from a transcriptionally active state to an arrested state that allows for removal of DNA lesions. This transition requires site-specific ubiquitylation of Pol II by the CRL4 CSA ubiquitin ligase, a process that is facilitated by ELOF1 in an unknown way. Using cryogenic electron microscopy, biochemical assays and cell biology approaches, we found that ELOF1 serves as an adaptor to stably position UVSSA and CRL4 CSA on arrested Pol II, leading to ligase neddylation and activation of Pol II ubiquitylation. In the presence of ELOF1, a transcription factor IIS (TFIIS)-like element in UVSSA gets ordered and extends through the Pol II pore, thus preventing reactivation of Pol II by TFIIS. Our results provide the structural basis for Pol II ubiquitylation and inactivation in TCR.
- Department of Molecular Biology, Max Planck Institute for Multidisciplinary Sciences, Göttingen, Germany.
Organizational Affiliation: