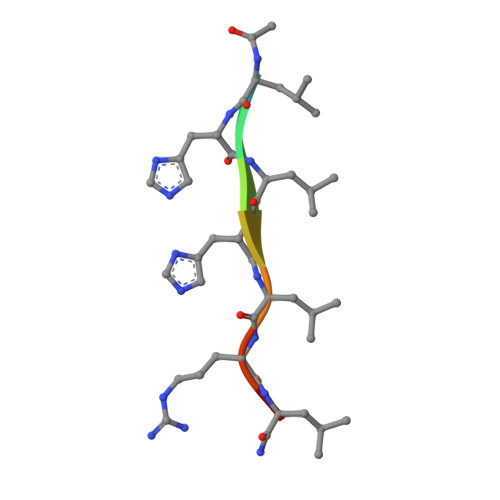
Cryo-EM structure of a catalytic amyloid fibril.
Heerde, T., Bansal, A., Schmidt, M., Fandrich, M.(2023) Sci Rep 13: 4070-4070
- PubMed: 36906667
- DOI: https://doi.org/10.1038/s41598-023-30711-y
- Primary Citation of Related Structures:
8B3A - PubMed Abstract:
Catalytic amyloid fibrils are novel types of bioinspired, functional materials that combine the chemical and mechanical robustness of amyloids with the ability to catalyze a certain chemical reaction. In this study we used cryo-electron microcopy to analyze the amyloid fibril structure and the catalytic center of amyloid fibrils that hydrolyze ester bonds. Our findings show that catalytic amyloid fibrils are polymorphic and consist of similarly structured, zipper-like building blocks that consist of mated cross-β sheets. These building blocks define the fibril core, which is decorated by a peripheral leaflet of peptide molecules. The observed structural arrangement differs from previously described catalytic amyloid fibrils and yielded a new model of the catalytic center.
- Institute of Protein Biochemistry, Ulm University, 89081, Ulm, Germany. thomas.heerde@uni-ulm.de.
Organizational Affiliation: