Mechanism of D-Cycloserine Inhibition of D-Amino Acid Transaminase from Haliscomenobacter hydrossis.
Bakunova, A.K., Matyuta, I.O., Nikolaeva, A.Y., Boyko, K.M., Popov, V.O., Bezsudnova, E.Y.(2023) Biochemistry (Mosc) 88: 687-697
- PubMed: 37331714
- DOI: https://doi.org/10.1134/S0006297923050115
- Primary Citation of Related Structures:
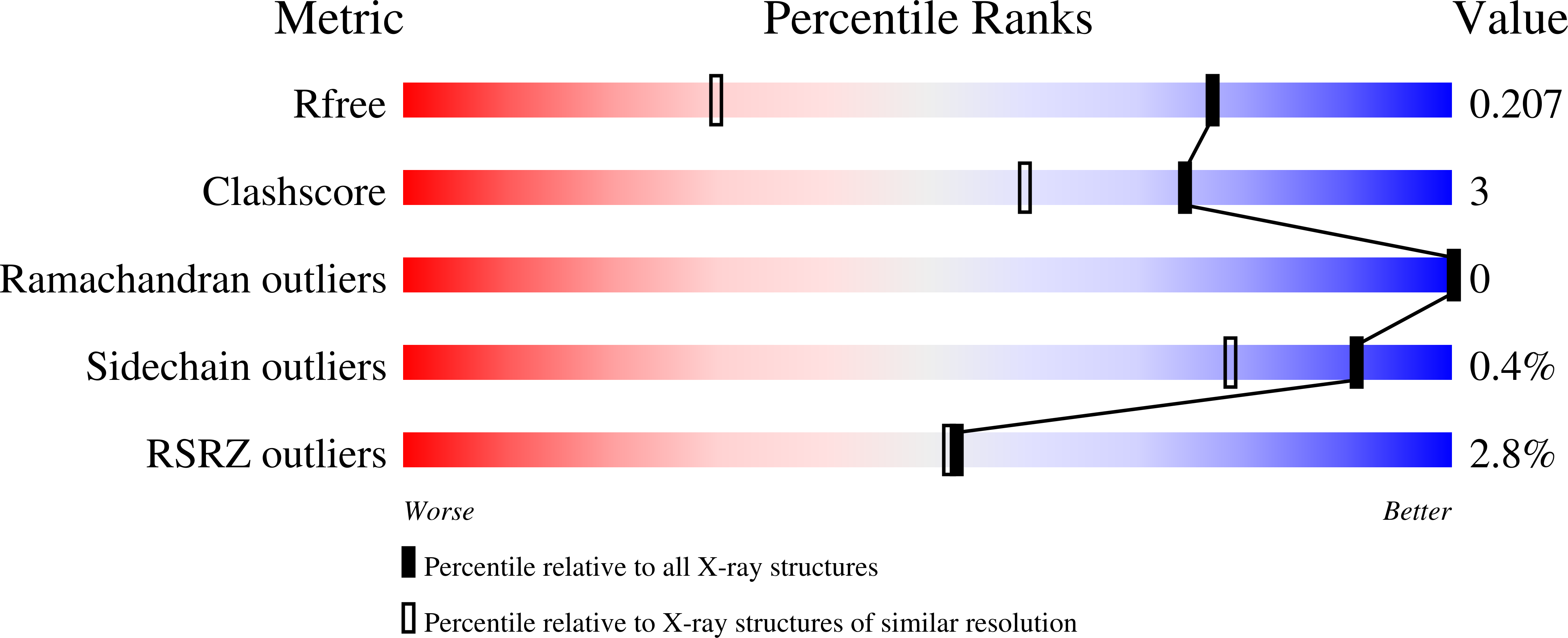
8AHU - PubMed Abstract:
D-cycloserine inhibits pyridoxal-5'-phosphate (PLP)-dependent enzymes. Inhibition effect depend on organization of the active site and mechanism of the catalyzed reaction. D-cycloserine interacts with the PLP form of the enzyme similarly to the substrate (amino acid), and this interaction is predominantly reversible. Several products of the interaction of PLP with D-cycloserine are known. For some enzymes formation of a stable aromatic product - hydroxyisoxazole-pyridoxamine-5'-phosphate at certain pH - leads to irreversible inhibition. The aim of this work was to study the mechanism of D-cycloserine inhibition of the PLP-dependent D-amino acid transaminase from Haliscomenobacter hydrossis. Spectral methods revealed several products of interaction of D-cycloserine with PLP in the active site of transaminase: oxime between PLP and β-aminooxy-D-alanine, ketimine between pyridoxamine-5'-phosphate and cyclic form of D-cycloserine, and pyridoxamine-5'-phosphate. Formation of hydroxyisoxazole-pyridoxamine-5'-phosphate was not observed. 3D structure of the complex with D-cycloserine was obtained using X-ray diffraction analysis. In the active site of transaminase, a ketimine adduct between pyridoxamine-5'-phosphate and D-cycloserine in the cyclic form was found. Ketimine occupied two positions interacting with different active site residues via hydrogen bonds. Using kinetic and spectral methods we have shown that D-cycloserine inhibition is reversible, and activity of the inhibited transaminase from H. hydrossis could be restored by adding excess of keto substrate or excess of cofactor. The obtained results confirm reversibility of the inhibition by D-cycloserine and interconversion of various adducts of D-cycloserine and PLP.
Organizational Affiliation:
Bach Institute of Biochemistry, Research Centre of Biotechnology, Russian Academy of Sciences, Moscow, 119071, Russia. a.bakunova@fbras.ru.