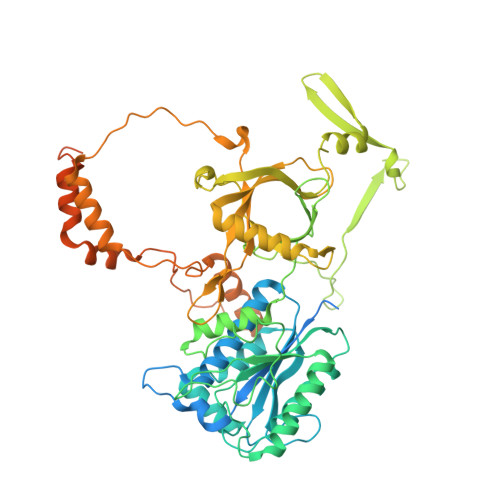
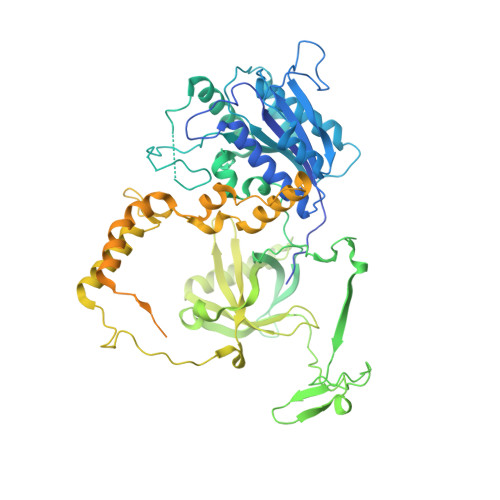
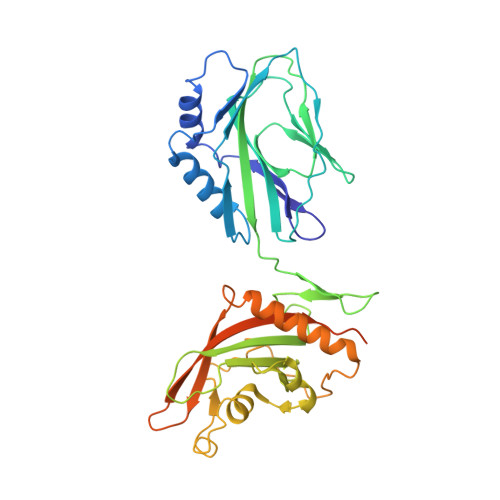
Structural basis for the inactivation of cytosolic DNA sensing by the vaccinia virus.
Rivera-Calzada, A., Arribas-Bosacoma, R., Ruiz-Ramos, A., Escudero-Bravo, P., Boskovic, J., Fernandez-Leiro, R., Oliver, A.W., Pearl, L.H., Llorca, O.(2022) Nat Commun 13: 7062-7062
- PubMed: 36400800
- DOI: https://doi.org/10.1038/s41467-022-34843-z
- Primary Citation of Related Structures:
8AG3, 8AG4, 8AG5 - PubMed Abstract:
Detection of cytosolic DNA is a central element of the innate immunity system against viral infection. The Ku heterodimer, a component of the NHEJ pathway of DNA repair in the nucleus, functions as DNA sensor that detects dsDNA of viruses that replicate in the cytoplasm. Vaccinia virus expresses two proteins, C4 and C16, that inactivate DNA sensing and enhance virulence. The structural basis for this is unknown. Here we determine the structure of the C16 - Ku complex using cryoEM. Ku binds dsDNA by a preformed ring but C16 sterically blocks this access route, abrogating binding to a dsDNA end and its insertion into DNA-PK, thereby averting signalling into the downstream innate immunity system. C4 replicates these activities using a domain with 54% identity to C16. Our results reveal how vaccinia virus subverts the capacity of Ku to recognize viral DNA.
- Spanish National Cancer Research Centre (CNIO), Melchor Fernández Almagro 3, 28029, Madrid, Spain.
Organizational Affiliation: