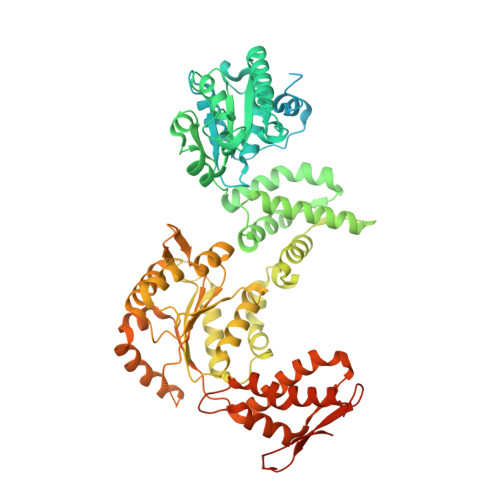
Structure of the drug target ClpC1 unfoldase in action provides insights on antibiotic mechanism of action.
Weinhaupl, K., Gragera, M., Bueno-Carrasco, M.T., Arranz, R., Krandor, O., Akopian, T., Soares, R., Rubin, E., Felix, J., Fraga, H.(2022) J Biological Chem 298: 102553-102553
- PubMed: 36208775
- DOI: https://doi.org/10.1016/j.jbc.2022.102553
- Primary Citation of Related Structures:
8A8U, 8A8V, 8A8W - PubMed Abstract:
The unfoldase ClpC1 is one of the most exciting drug targets against tuberculosis. This AAA+ unfoldase works in cooperation with the ClpP1P2 protease and is the target of at least four natural product antibiotics: cyclomarin, ecumicin, lassomycin, and rufomycin. Although these molecules are promising starting points for drug development, their mechanisms of action remain largely unknown. Taking advantage of a middle domain mutant, we determined the first structure of Mycobacterium tuberculosis ClpC1 in its apo, cyclomarin-, and ecumicin-bound states via cryo-EM. The obtained structure displays features observed in other members of the AAA+ family and provides a map for further drug development. While the apo and cyclomarin-bound structures are indistinguishable and have N-terminal domains that are invisible in their respective EM maps, around half of the ecumicin-bound ClpC1 particles display three of their six N-terminal domains in an extended conformation. Our structural observations suggest a mechanism where ecumicin functions by mimicking substrate binding, leading to ATPase activation and changes in protein degradation profile.
- i3S, Instituto de Investigacao e Inovacao em Saude, Universidade do Porto, Porto, Portugal.
Organizational Affiliation: