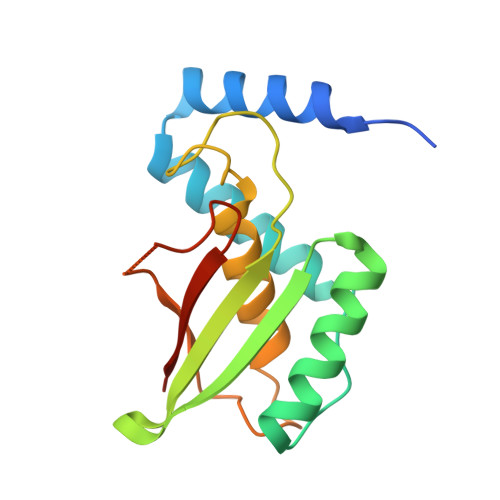
Bacterial crystalline cellulose secretion via a supramolecular BcsHD scaffold.
Abidi, W., Decossas, M., Torres-Sanchez, L., Puygrenier, L., Letoffe, S., Ghigo, J.M., Krasteva, P.V.(2022) Sci Adv 8: eadd1170-eadd1170
- PubMed: 36525496
- DOI: https://doi.org/10.1126/sciadv.add1170
- Primary Citation of Related Structures:
7ZZQ, 7ZZY - PubMed Abstract:
Cellulose, the most abundant biopolymer on Earth, is not only the predominant constituent of plants but also a key extracellular polysaccharide in the biofilms of many bacterial species. Depending on the producers, chemical modifications, and three-dimensional assemblies, bacterial cellulose (BC) can present diverse degrees of crystallinity. Highly ordered, or crystalline, cellulose presents great economical relevance due to its ever-growing number of biotechnological applications. Even if some acetic acid bacteria have long been identified as BC superproducers, the molecular mechanisms determining the secretion of crystalline versus amorphous cellulose remain largely unknown. Here, we present structural and mechanistic insights into the role of the accessory subunits BcsH (CcpAx) and BcsD (CesD) that determine crystalline BC secretion in the Gluconacetobacter lineage. We show that oligomeric BcsH drives the assembly of BcsD into a supramolecular cytoskeletal scaffold that likely stabilizes the cellulose-extruding synthase nanoarrays through an unexpected inside-out mechanism for secretion system assembly.
- Université de Bordeaux, CNRS, Bordeaux INP, CBMN, UMR 5248, Pessac, France.
Organizational Affiliation: