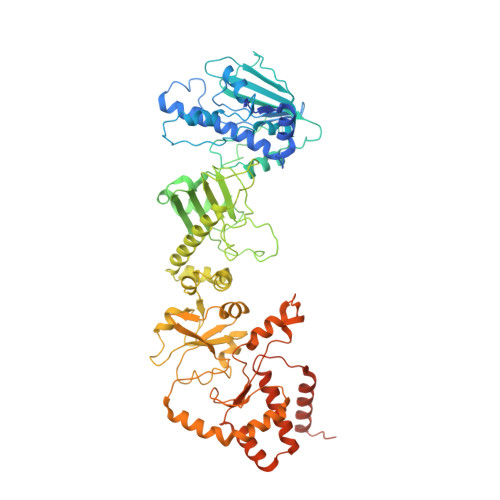
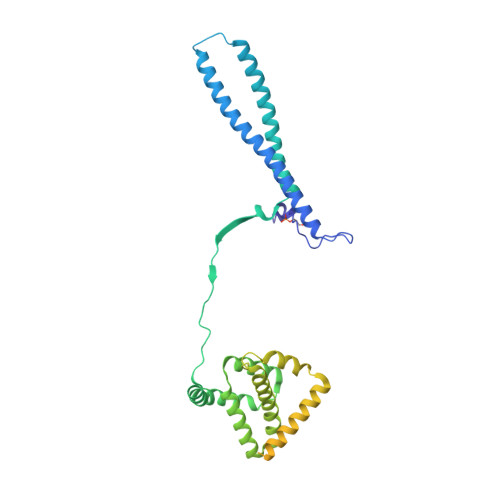
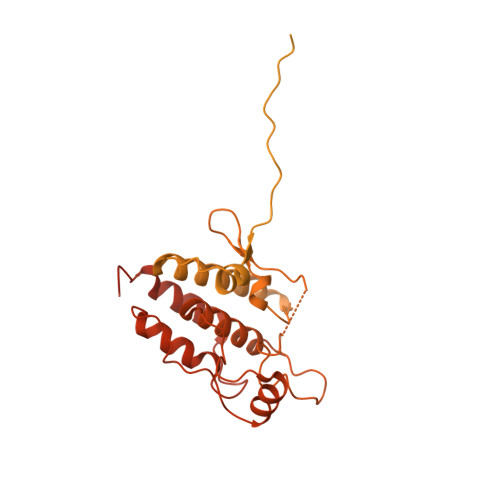
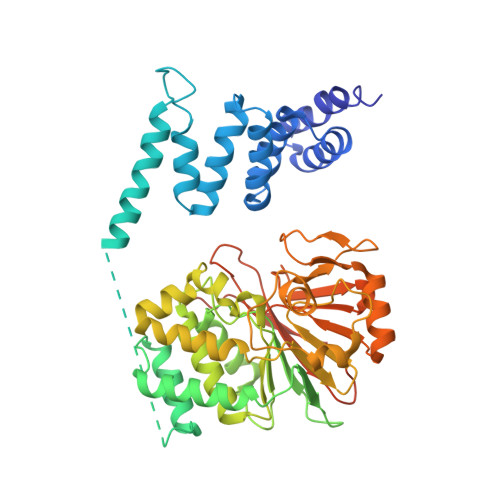
HSP90-CDC37-PP5 forms a structural platform for kinase dephosphorylation.
Oberoi, J., Guiu, X.A., Outwin, E.A., Schellenberger, P., Roumeliotis, T.I., Choudhary, J.S., Pearl, L.H.(2022) Nat Commun 13: 7343-7343
- PubMed: 36446791
- DOI: https://doi.org/10.1038/s41467-022-35143-2
- Primary Citation of Related Structures:
7ZR0, 7ZR5, 7ZR6 - PubMed Abstract:
Activation of client protein kinases by the HSP90 molecular chaperone system is affected by phosphorylation at multiple sites on HSP90, the kinase-specific co-chaperone CDC37, and the kinase client itself. Removal of regulatory phosphorylation from client kinases and their release from the HSP90-CDC37 system depends on the Ser/Thr phosphatase PP5, which associates with HSP90 via its N-terminal TPR domain. Here, we present the cryoEM structure of the oncogenic protein kinase client BRAF V600E bound to HSP90-CDC37, showing how the V600E mutation favours BRAF association with HSP90-CDC37. Structures of HSP90-CDC37-BRAF V600E complexes with PP5 in autoinhibited and activated conformations, together with proteomic analysis of its phosphatase activity on BRAF V600E and CRAF, reveal how PP5 is activated by recruitment to HSP90 complexes. PP5 comprehensively dephosphorylates client proteins, removing interaction sites for regulatory partners such as 14-3-3 proteins and thus performing a 'factory reset' of the kinase prior to release.
- Genome Damage and Stability Centre, School of Life Sciences, University of Sussex, Falmer, Brighton, BN1 9RQ, UK.
Organizational Affiliation: