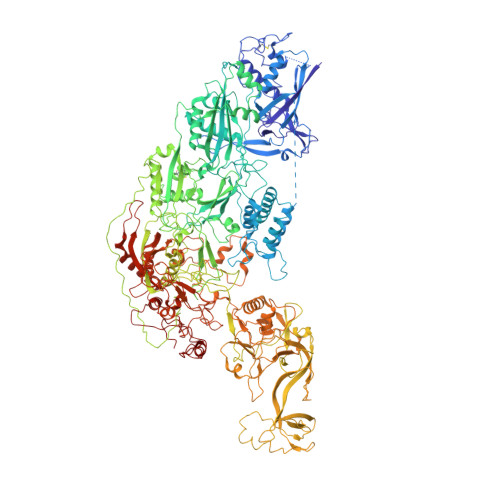
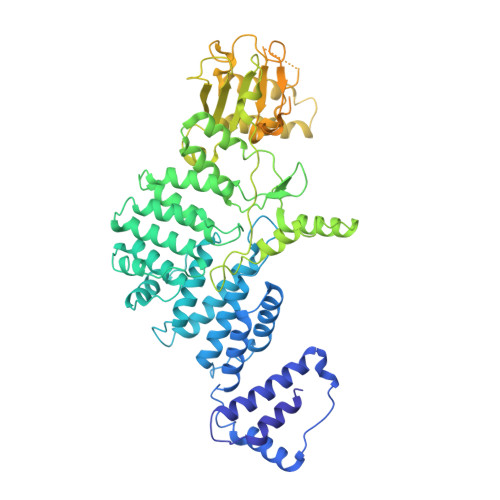
Cryo-EM structure and protease activity of the type III-E CRISPR-Cas effector.
Huo, Y., Zhao, H., Dong, Q., Jiang, T.(2023) Nat Microbiol 8: 522-532
- PubMed: 36702942
- DOI: https://doi.org/10.1038/s41564-022-01316-4
- Primary Citation of Related Structures:
7YN9, 7YNA, 7YNB, 7YNC, 7YND - PubMed Abstract:
The recently discovered type III-E CRISPR-Cas effector Cas7-11 shows promise when used as an RNA manipulation tool, but its structure and the mechanisms underlying its function remain unclear. Here we present four cryo-EM structures of Desulfonema ishimotonii Cas7-11-crRNA complex in pre-target and target RNA-bound states, and the cryo-EM structure of DiCas7-11-crRNA bound to its accessory protein DiCsx29. These data reveal structural elements for pre-crRNA processing, target RNA cleavage and regulation. Moreover, a 3' seed region of crRNA is involved in regulating RNA cleavage activity of DiCas7-11-crRNA-Csx29. Our analysis also shows that both the minimal mismatch of 4 nt to the 5' handle of crRNA and the minimal matching of the first 12 nt of the spacer by the target RNA are essential for triggering the protease activity of DiCas7-11-crRNA-Csx29 towards DiCsx30. Taken together, we propose that target RNA recognition and cleavage regulate and fine-tune the protease activity of DiCas7-11-crRNA-Csx29, thus preventing auto-immune responses.
- National Laboratory of Macromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing, China. huoyangao@ibp.ac.cn.
Organizational Affiliation: