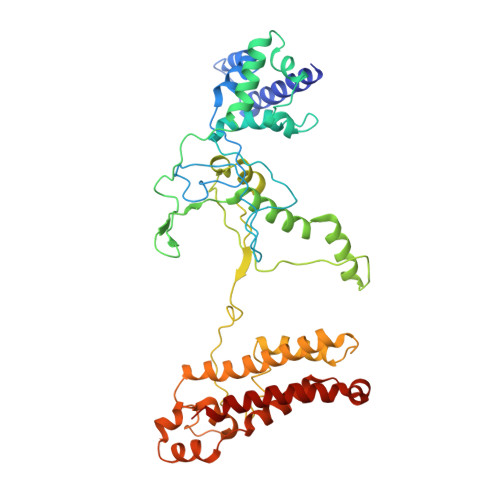
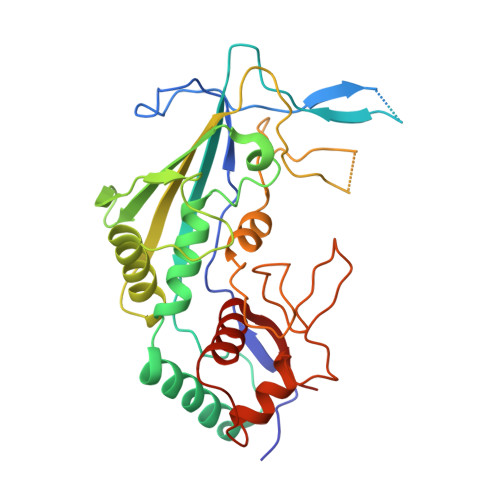
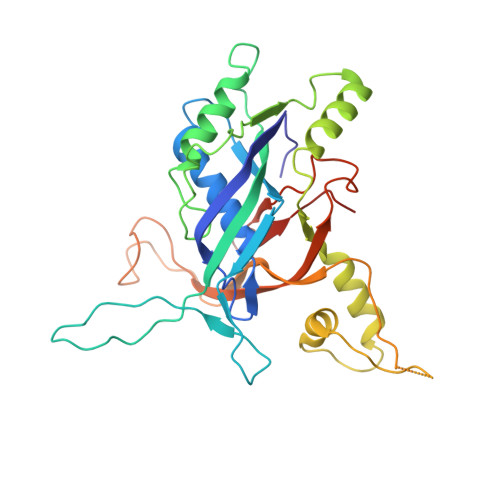
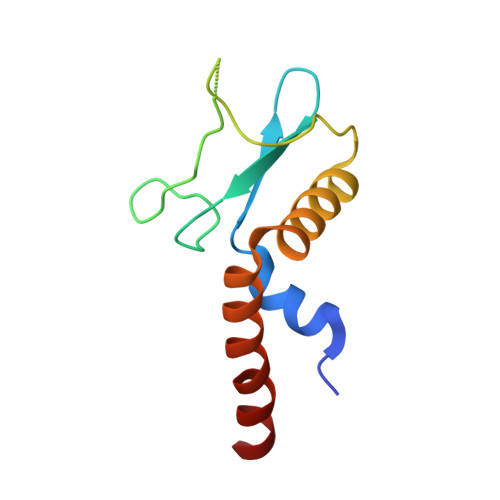
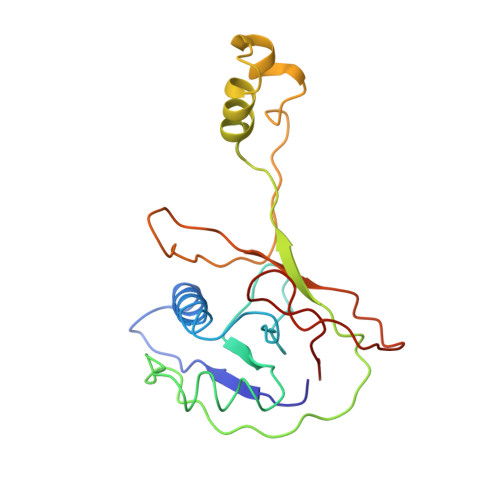
Anti-CRISPR protein AcrIF4 inhibits the type I-F CRISPR-Cas surveillance complex by blocking nuclease recruitment and DNA cleavage.
Gao, Z., Zhang, L., Ge, Z., Wang, H., Yue, Y., Jiang, Z., Wang, X., Xu, C., Zhang, Y., Yang, M., Feng, Y.(2022) J Biological Chem 298: 102575-102575
- PubMed: 36209819
- DOI: https://doi.org/10.1016/j.jbc.2022.102575
- Primary Citation of Related Structures:
7YHS - PubMed Abstract:
The clustered regularly interspaced short palindromic repeats (CRISPR)-Cas system provides prokaryotes with protection against mobile genetic elements such as phages. In turn, phages deploy anti-CRISPR (Acr) proteins to evade this immunity. AcrIF4, an Acr targeting the type I-F CRISPR-Cas system, has been reported to bind the crRNA-guided surveillance (Csy) complex. However, it remains controversial whether AcrIF4 inhibits target DNA binding to the Csy complex. Here, we present structural and mechanistic studies into AcrIF4, exploring its unique anti-CRISPR mechanism. While the Csy-AcrIF4 complex displays decreased affinity for target DNA, it is still able to bind the DNA. Our structural and functional analyses of the Csy-AcrIF4-dsDNA complex revealed that AcrIF4 binding prevents rotation of the helical bundle of the Cas8f subunit induced by dsDNA binding, therefore resulting in failure of nuclease Cas2/3 recruitment and DNA cleavage. Overall, our study provides an interesting example of attack on the nuclease recruitment event by an Acr, but not conventional mechanisms of blocking binding of target DNA.
- Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing Key Laboratory of Bioprocess, State Key Laboratory of Chemical Resource Engineering, College of Life Science and Technology, Beijing University of Chemical Technology, Beijing, China.
Organizational Affiliation: