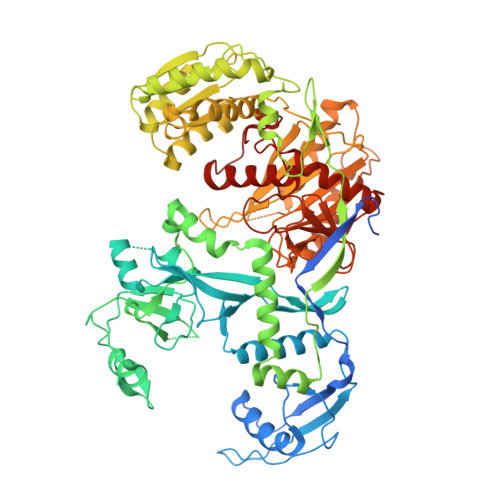
Mammalian PIWI-piRNA-target complexes reveal features for broad and efficient target silencing.
Li, Z., Li, Z., Zhang, Y., Zhou, L., Xu, Q., Li, L., Zeng, L., Xue, J., Niu, H., Zhong, J., Yu, Q., Li, D., Gui, M., Huang, Y., Tu, S., Zhang, Z., Song, C.Q., Wu, J., Shen, E.Z.(2024) Nat Struct Mol Biol
- PubMed: 38658622
- DOI: https://doi.org/10.1038/s41594-024-01287-6
- Primary Citation of Related Structures:
7YFQ, 7YFX, 7YFY, 7YG6, 7YGN - PubMed Abstract:
The PIWI-interacting RNA (piRNA) pathway is an adaptive defense system wherein piRNAs guide PIWI family Argonaute proteins to recognize and silence ever-evolving selfish genetic elements and ensure genome integrity. Driven by this intensive host-pathogen arms race, the piRNA pathway and its targeted transposons have coevolved rapidly in a species-specific manner, but how the piRNA pathway adapts specifically to target silencing in mammals remains elusive. Here, we show that mouse MILI and human HILI piRNA-induced silencing complexes (piRISCs) bind and cleave targets more efficiently than their invertebrate counterparts from the sponge Ephydatia fluviatilis. The inherent functional differences comport with structural features identified by cryo-EM studies of piRISCs. In the absence of target, MILI and HILI piRISCs adopt a wider nucleic-acid-binding channel and display an extended prearranged piRNA seed as compared with EfPiwi piRISC, consistent with their ability to capture targets more efficiently than EfPiwi piRISC. In the presence of target, the seed gate-which enforces seed-target fidelity in microRNA RISC-adopts a relaxed state in mammalian piRISC, revealing how MILI and HILI tolerate seed-target mismatches to broaden the target spectrum. A vertebrate-specific lysine distorts the piRNA seed, shifting the trajectory of the piRNA-target duplex out of the central cleft and toward the PAZ lobe. Functional analyses reveal that this lysine promotes target binding and cleavage. Our study therefore provides a molecular basis for the piRNA targeting mechanism in mice and humans, and suggests that mammalian piRNA machinery can achieve broad target silencing using a limited supply of piRNA species.
- School of Basic Medical Sciences, Fudan University, Shanghai, China.
Organizational Affiliation: