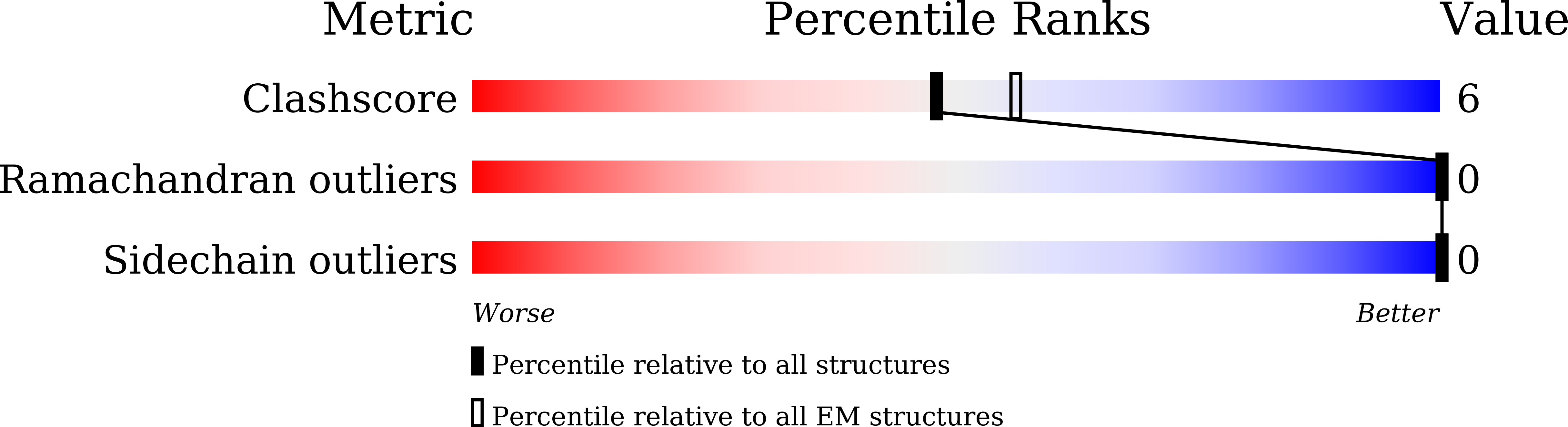Conformational change of alpha-synuclein fibrils in cerebrospinal fluid from different clinical phases of Parkinson's disease.
Fan, Y., Sun, Y., Yu, W., Tao, Y., Xia, W., Liu, Y., Zhao, Q., Tang, Y., Sun, Y., Liu, F., Cao, Q., Wu, J., Liu, C., Wang, J., Li, D.(2023) Structure 31: 78
- PubMed: 36513068
- DOI: https://doi.org/10.1016/j.str.2022.11.013
- Primary Citation of Related Structures:
7V47, 7V48, 7V49, 7XO0, 7XO1, 7XO2, 7XO3, 8H03, 8H04, 8H05 - PubMed Abstract:
α-Synuclein (α-syn) has been shown to form various conformational fibrils associated with different synucleinopathies. But whether the conformation of α-syn fibrils changes during disease progression is unclear. Here, we amplified α-syn aggregates from the cerebrospinal fluid (CSF) of patients with Parkinson's disease (PD) staged in preclinical PD (pre-PD), middle- to late-stage PD (mid-PD), and late-stage PD (late-PD). Our results show that α-syn fibrils derived from the late-PD patient are most potent in inducing endogenous α-syn aggregation in primary neurons, followed by the mid-PD and pre-PD fibrils. By using cryo-electron microscopy, we further determined the high-resolution structures of the CSF-amplified fibrils. The structures exhibit remarkable differences in a minor but significant population of conformational species in different staged samples. Our work demonstrates structural and pathological differences between α-syn fibrils derived from PD patients at a spectrum of clinical stages, which suggests potential conformational transition of α-syn fibrils during the progression of PD.
- Department of Neurology and National Research Center for Aging and Medicine & National Center for Neurological Disorders, State Key Laboratory of Medical Neurobiology, Huashan Hospital, Fudan University, Shanghai 200040, China.
Organizational Affiliation: