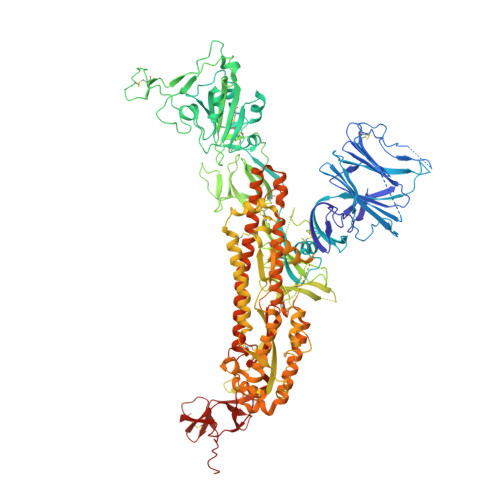
Biparatopic antibody BA7208/7125 effectively neutralizes SARS-CoV-2 variants including Omicron BA.1-BA.5.
Wang, Y., Yan, A., Song, D., Dong, C., Rao, M., Gao, Y., Qi, R., Ma, X., Wang, Q., Xu, H., Liu, H., Han, J., Duan, M., Liu, S., Yu, X., Zong, M., Feng, J., Jiao, J., Zhang, H., Li, M., Yu, B., Wang, Y., Meng, F., Ni, X., Li, Y., Shen, Z., Sun, B., Shao, X., Zhao, H., Zhao, Y., Li, R., Zhang, Y., Du, G., Lu, J., You, C., Jiang, H., Zhang, L., Wang, L., Dou, C., Liu, Z., Zhao, J.(2023) Cell Discov 9: 3-3
- PubMed: 36609558
- DOI: https://doi.org/10.1038/s41421-022-00509-9
- Primary Citation of Related Structures:
7XCZ, 7XDA, 7XDB, 7XDK, 7XDL - PubMed Abstract:
SARS-CoV-2 Omicron subvariants have demonstrated extensive evasion from monoclonal antibodies (mAbs) developed for clinical use, which raises an urgent need to develop new broad-spectrum mAbs. Here, we report the isolation and analysis of two anti-RBD neutralizing antibodies BA7208 and BA7125 from mice engineered to produce human antibodies. While BA7125 showed broadly neutralizing activity against all variants except the Omicron sublineages, BA7208 was potently neutralizing against all tested SARS-CoV-2 variants (including Omicron BA.1-BA.5) except Mu. By combining BA7208 and BA7125 through the knobs-into-holes technology, we generated a biparatopic antibody BA7208/7125 that was able to neutralize all tested circulating SARS-CoV-2 variants. Cryo-electron microscopy structure of these broad-spectrum antibodies in complex with trimeric Delta and Omicron spike indicated that the contact residues are highly conserved and had minimal interactions with mutational residues in RBD of current variants. In addition, we showed that administration of BA7208/7125 via the intraperitoneal, intranasal, or aerosol inhalation route showed potent therapeutic efficacy against Omicron BA.1 and BA.2 in hACE2-transgenic and wild-type mice and, separately, effective prophylaxis. BA7208/7125 thus has the potential to be an effective candidate as an intervention against COVID-19.
- State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Health, the First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, China.
Organizational Affiliation: