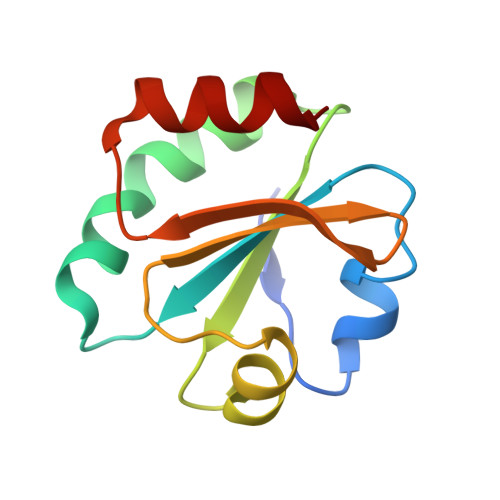
High-resolution crystal structure of Acinetobacter baumannii thioredoxin 1.
Chang, Y.J., Park, H.H.(2022) Biochem Biophys Res Commun 608: 1-7
- PubMed: 35378360
- DOI: https://doi.org/10.1016/j.bbrc.2022.03.134
- Primary Citation of Related Structures:
7XAO - PubMed Abstract:
Thioredoxin (Trx) is a central component of the redox control system that maintains the redox homeostasis critical for organism survival. Owing to its central role in survival, Trx is a prospective target for novel antimicrobial agents. Herein, we report a 1.45 Å high-resolution structure of Trx1 of Acinetobacter baumannii (abTrx1), an antibiotic-resistant pathogenic superbug. Although abTrx1 exhibited the canonical Trx fold, which consists of a four-stranded β-sheet surrounded by four α-helices, structural differences were detected in the loop forming the C-X-X-C redox center and the C-terminal. The unique CAPC sequence of the C-X-X-C motif in the abTrx1 redox center was characterized by mutagenesis. This study contributes to the field of drug designing against superbugs.
- College of Pharmacy, Chung-Ang University, Seoul, 06974, Republic of Korea; Department of Global Innovative Drugs, Graduate School of Chung-Ang University, Seoul, 06974, Republic of Korea.
Organizational Affiliation: