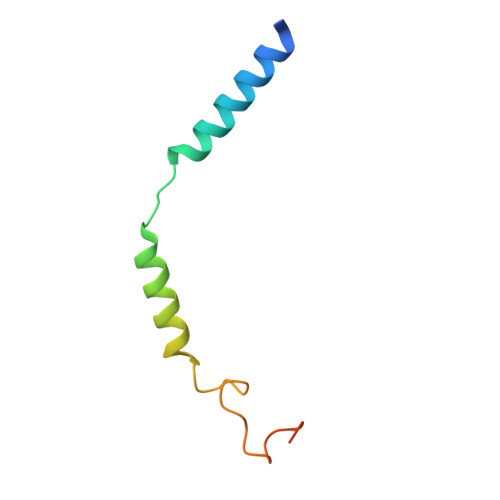
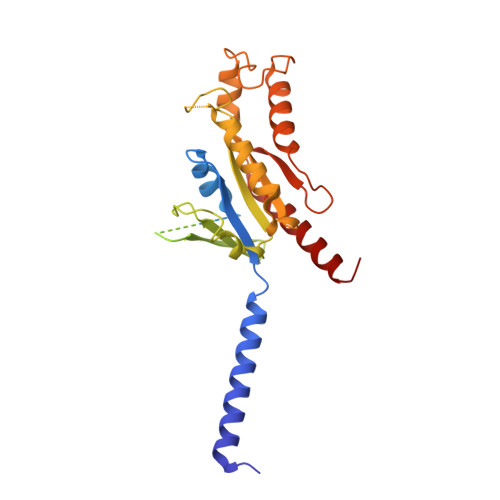
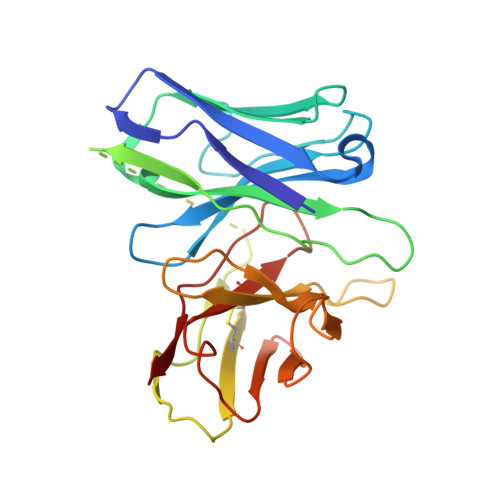
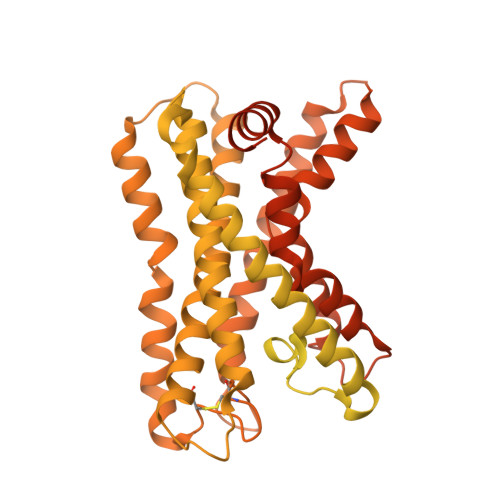
Structural basis of adhesion GPCR GPR110 activation by stalk peptide and G-proteins coupling.
Zhu, X., Qian, Y., Li, X., Xu, Z., Xia, R., Wang, N., Liang, J., Yin, H., Zhang, A., Guo, C., Wang, G., He, Y.(2022) Nat Commun 13: 5513-5513
- PubMed: 36127364
- DOI: https://doi.org/10.1038/s41467-022-33173-4
- Primary Citation of Related Structures:
7WXU, 7WXW, 7WY0, 7WZ7, 7X2V - PubMed Abstract:
Adhesion G protein-coupled receptors (aGPCRs) are keys of many physiological events and attractive targets for various diseases. aGPCRs are also known to be capable of self-activation via an autoproteolysis process that removes the inhibitory GAIN domain on the extracellular side of receptor and releases a stalk peptide to bind and activate the transmembrane side of receptor. However, the detailed mechanism of aGPCR activation remains elusive. Here, we report the cryo-electron microscopy structures of GPR110 (ADGRF1), a member of aGPCR, in complex with G q , G s , G i , G 12 and G 13. The structures reveal distinctive ligand engaging model and activation conformations of GPR110. The structures also unveil the rarely explored GPCR/G 12 and GPCR/G 13 engagements. A comparison of G q , G s , G i , G 12 and G 13 engagements with GPR110 reveals details of G-protein engagement, including a dividing point at the far end of the alpha helix 5 (αH5) of Gα subunit that separates G q /G s engagements from G i /G 12 /G 13 engagements. This is also where G q /G s bind the receptor through both hydrophobic and polar interaction, while G i /G 12 /G 13 engage receptor mainly through hydrophobic interaction. We further provide physiological evidence of GPR110 activation via stalk peptide. Taken together, our study fills the missing information of GPCR/G-protein engagement and provides a framework for understanding aGPCR activation and GPR110 signaling.
- Laboratory of Receptor Structure and Signaling, HIT Center for Life Sciences, Harbin Institute of Technology, Harbin, 150001, China.
Organizational Affiliation: