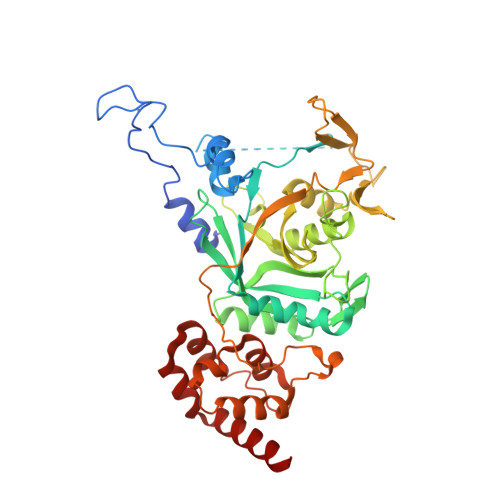
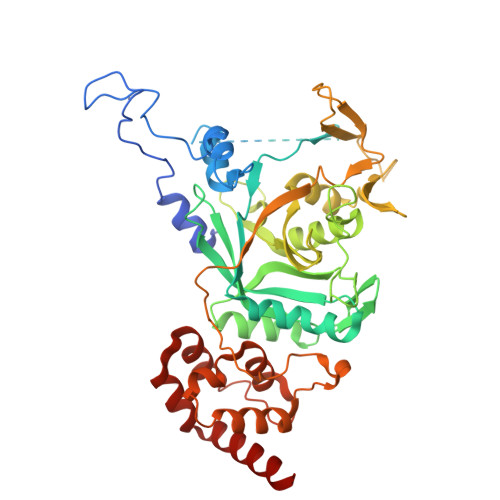
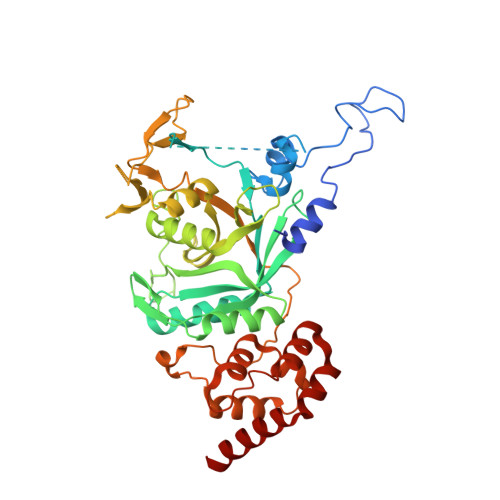
Structural basis for the interaction between human Npl4 and Npl4-binding motif of human Ufd1.
Nguyen, T.Q., My Le, L.T., Kim, D.H., Ko, K.S., Lee, H.T., Kim Nguyen, Y.T., Kim, H.S., Han, B.W., Kang, W., Yang, J.K.(2022) Structure 30: 1530-1537.e3
- PubMed: 36087575
- DOI: https://doi.org/10.1016/j.str.2022.08.005
- Primary Citation of Related Structures:
7WWP, 7WWQ - PubMed Abstract:
The heterodimer of human ubiquitin fusion degradation 1 (hUfd1) and human nuclear protein localization 4 (hNpl4) is a major cofactor of human p97 adenosine triphosphatase (ATPase). The p97-Ufd1-Npl4 complex translocates the ubiquitin-conjugated proteins from the endoplasmic reticulum membrane to the cytoplasm. Ubiquitinated proteins are then degraded by the proteasome. The structures of Npl4 and Ufd1-Npl4 (UN) complex in Saccharomyces cerevisiae have been recently reported; however, the structures of hNpl4 and the human UN complex remain unknown. Here, we report the crystal structures of the human UN complex at a resolution of 2.7 Å and hNpl4 at a resolution of 3.0 Å. We also present atomic details and characterization of the human UN complex. Crystallographic studies and site-directed mutagenesis of the hUfd1 residues involved in the interaction with hNpl4 revealed the atomic details of the two proteins.
Organizational Affiliation:
Department of Chemistry, College of Natural Sciences, Soongsil University, Seoul 06978, Republic of Korea.